Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma
- PMID: 33777728
- PMCID: PMC7987916
- DOI: 10.3389/fonc.2021.546586
Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma
Abstract
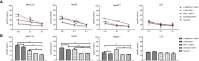
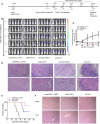
T cells expressing chimeric antigen receptors, especially CD19 CAR-T cells have exhibited effective antitumor activities in B cell malignancies, but due to several factors such as antigen escape effects and tumor microenvironment, their curative potential in hepatocellular carcinoma has not been encouraging. To reduce the antigen escape risk of hepatocellular carcinoma, this study was to design and construct a bispecific CAR targeting c-Met and PD-L1. c-Met/PD-L1 CAR-T cells were obtained by lentiviral transfection, and the transfection efficiency was monitored by flow cytometry analysis. LDH release assays were used to elucidate the efficacy of c-Met/PD-L1 CAR-T cells on hepatocellular carcinoma cells. In addition, xenograft models bearing human hepatocellular carcinoma were constructed to detect the antitumor effect of c-Met/PD-L1 CAR-T cells in vivo. The results shown that this bispecific CAR was manufactured successfully, T cells modified with this bispecific CAR demonstrated improved antitumor activities against c-Met and PD-L1 positive hepatocellular carcinoma cells when compared with those of monovalent c-Met CAR-T cells or PD-L1 CAR-T cells but shown no distinct cytotoxicity on hepatocytes in vitro. In vivo experiments shown that c-Met/PD-L1 CAR-T cells significantly inhibited tumor growth and improve survival persistence compared with other groups. These results suggested that the design of single-chain, bi-specific c-Met/PD-L1 CAR-T is more effective than that of monovalent c-Met CAR-T for the treatment of hepatocellular carcinoma., and this bi-specific c-Met/PD-L1 CAR is rational and implementable with current T-cell engineering technology.
Keywords: CAR-T cell; PD-L1; antigen relapse effects; c-Met; hepatocellular carcinoma.
Copyright © 2021 Jiang, Li, Guo, Wang, Jia, shi, Yang, Jiao, Wei, Feng, Tang and Ji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. . T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (2015) 385:517–28. 10.1016/S0140-6736(14)61403-3 - DOI - PMC - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. . Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol (2015) 33:540–9. 10.1200/JCO.2014.56.2025 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

