Prognosis and Genomic Landscape of Liver Metastasis in Patients With Breast Cancer
- PMID: 33777740
- PMCID: PMC7991092
- DOI: 10.3389/fonc.2021.588136
Prognosis and Genomic Landscape of Liver Metastasis in Patients With Breast Cancer
Abstract
Objective: The prognosis of breast cancer liver metastasis (BCLM) is poor, and its molecular mechanism is unclear. We aimed to determine the factors that affect the prognosis of patients with BCLM and investigate the genomic landscape of liver metastasis (LM).
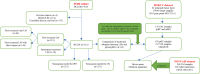
Methods: We described the prognosis of patients with BCLM and focused on prognosis prediction for these patients based on clinicopathological factors. Nomogram models were constructed for progression-free survival (PFS) and overall survival (OS) by using a cohort of 231 patients with BCLM who underwent treatment at Shandong Cancer Hospital and Institute (SCHI). We explored the molecular mechanism of LM and constructed driver genes, mutation signatures by using a targeted sequencing dataset of 217 samples of LM and 479 unpaired samples of primary breast cancer (pBC) from Memorial Sloan Kettering Cancer Center (MSKCC).
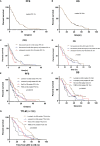
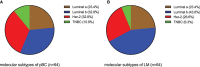
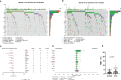
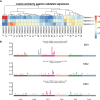
Results: The median follow-up time for 231 patients with BCLM in the SCHI cohort was 46 months. The cumulative incidence of LM at 1, 2, and 5 years was 17.5%, 45.0%, and 86.8%, respectively. The median PFS and OS were 7 months (95% CI, 6-8) and 22 months (95% CI, 19-25), respectively. The independent factors that increased the progression risk of patients with LM were Karnofsky performance status (KPS) ≤ 80, TNBC subtype, grade III, increasing trend of CA153, and disease-free interval (DFS) ≤ 1 year. Simultaneously, the independent factors that increased the mortality risk of patients with LM were Ki-67 ≥ 30%, grade III, increasing trend of CA153, pain with initial LM, diabetes, and DFI ≤ 1 year. In the MSKCC dataset, the LM driver genes were ESR1, AKT1, ERBB2, and FGFR4, and LM matched three prominent mutation signatures: APOBEC cytidine deaminase, ultraviolet exposure, and defective DNA mismatch repair.
Conclusion: This study systematically describes the survival prognosis and characteristics of LM from the clinicopathological factors to the genetic level. These results not only enable clinicians to assess the risk of disease progression in patients with BCLM to optimize treatment options, but also help us better understand the underlying mechanisms of tumor metastasis and evolution and provide new therapeutic targets with potential benefits for drug-resistant patients.
Keywords: breast cancer; genomic landscape; liver metastasis; nomogram model; prognosis.
Copyright © 2021 Tian, Liu, Wang and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

