The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy
- PMID: 33777750
- PMCID: PMC7991834
- DOI: 10.3389/fonc.2021.610303
The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy
Abstract
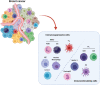
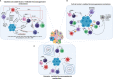
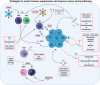
Breast cancer progression is a complex process controlled by genetic and epigenetic factors that coordinate the crosstalk between tumor cells and the components of tumor microenvironment (TME). Among those, the immune cells play a dual role during cancer onset and progression, as they can protect from tumor progression by killing immunogenic neoplastic cells, but in the meanwhile can also shape tumor immunogenicity, contributing to tumor escape. The complex interplay between cancer and the immune TME influences the outcome of immunotherapy and of many other anti-cancer therapies. Herein, we present an updated view of the pro- and anti-tumor activities of the main immune cell populations present in breast TME, such as T and NK cells, myeloid cells, innate lymphoid cells, mast cells and eosinophils, and of the underlying cytokine-, cell-cell contact- and microvesicle-based mechanisms. Moreover, current and novel therapeutic options that can revert the immunosuppressive activity of breast TME will be discussed. To this end, clinical trials assessing the efficacy of CAR-T and CAR-NK cells, cancer vaccination, immunogenic cell death-inducing chemotherapy, DNA methyl transferase and histone deacetylase inhibitors, cytokines or their inhibitors and other immunotherapies in breast cancer patients will be reviewed. The knowledge of the complex interplay that elapses between tumor and immune cells, and of the experimental therapies targeting it, would help to develop new combination treatments able to overcome tumor immune evasion mechanisms and optimize clinical benefit of current immunotherapies.
Keywords: breast cancer; cancer immunotherapy; immune checkpoint inhibitors (ICI); immunosuppression; tumor microenvironment (TME).
Copyright © 2021 Salemme, Centonze, Cavallo, Defilippi and Conti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

