Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center
- PMID: 33777810
- PMCID: PMC7991747
- DOI: 10.3389/fonc.2021.649209
Outcomes of Adults With Relapsed/Refractory Acute Myeloid Leukemia Treated With Venetoclax Plus Hypomethylating Agents at a Comprehensive Cancer Center
Abstract
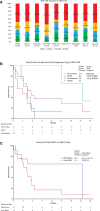
Relapsed/refractory acute myeloid leukemia (AML) is a devastating disease with a poor prognosis and represents a major unmet medical need. We report on a real-world academic center experience of treating 25 patients with relapsed/refractory AML using venetoclax in combination with decitabine or azacitidine, which is not otherwise widely evaluated in the current literature. Our patients come from a large, socioeconomically and geographically diverse area including the majority of Northern California. Most had ELN Adverse Risk (52%) or Intermediate Risk (44%) AML, and most had an ECOG Performance Status of 1 (64%). Over half (52%) had prior hypomethylating agent exposure, and 40% had Secondary AML. We observed an overall response rate of 52%, with eight patients (32%) achieving composite complete remission. Median overall survival was 5.5 months, and for patients achieving composite complete remission this was 21.6 months. One-year estimated overall survival was 38%. Three patients were able to proceed directly to stem cell transplant for consolidation, and all three were alive at last follow-up, ranging 13.8-24.0 months. We found venetoclax in combination with hypomethylating agents to be well tolerated and potentially efficacious in securing long-term remissions for patients with relapsed/refractory AML.
Keywords: acute myeloid leukemia; hypomethylating agent; real-world data; relapsed/refractory; venetoclax (BCL-2 inhibitor).
Copyright © 2021 Tenold, Moskoff, Benjamin, Hoeg, Rosenberg, Abedi, Tuscano and Jonas.
Conflict of interest statement
AR has received research funding from Amgen. He has participated in Speakers Bureaus for Janssen and Millenium-Takeda. He has served in a consulting/advisory role for Seattle Genetics and Karyopharm. MA has participated in Speakers Bureaus for AbbVie, Celgene, BMS, and Gilead. JT has received research funding from Celgene, Novartis, Achrotech, Pharmacyclics, Genentech, and Takeda. He has received honoraria from Celgene, Amgen and Seattle Genetics. Dr. Jonas has served in a consulting/advisory role for AbbVie, Amgen, Celgene, Genentech/Roche, GlycoMimetics, Jazz, Takeda, Tolero, and Treadwell. He has received travel support from AbbVie, Amgen, and GlycoMimetics. He has received research funding to his institution from 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, Celgene, Daiichi Sankyo, Esanex, F. Hoffmann-La Roche, Forma, Genentech/Roche, GlycoMimetics, Hanmi, Incyte, Jazz, LP Therapeutics, Pfizer, Pharmacyclics, and Sigma Tau. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- National Caner Institute . SEER. SEER Cancer Stat Facts: Acute Myeloid Leukemia. https://seer.cancer.gov/statfacts/html/amyl.html Accessed June 27, 2020.
LinkOut - more resources
Full Text Sources
Other Literature Sources

