Protective Effects of Dexmedetomidine on the Vascular Endothelial Barrier Function by Inhibiting Mitochondrial Fission via ER/Mitochondria Contact
- PMID: 33777946
- PMCID: PMC7991806
- DOI: 10.3389/fcell.2021.636327
Protective Effects of Dexmedetomidine on the Vascular Endothelial Barrier Function by Inhibiting Mitochondrial Fission via ER/Mitochondria Contact
Abstract
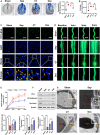
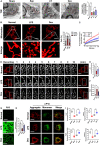
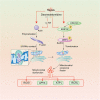
The damage of vascular endothelial barrier function induced by sepsis is critical in causing multiple organ dysfunctions. Previous studies showed that dexmedetomidine (Dex) played a vital role in protecting organ functions. However, whether Dex participates in protecting vascular leakage of sepsis and the associated underlying mechanism remains unknown yet. We used cecal ligation and puncture induced septic rats and lipopolysaccharide stimulated vascular endothelial cells (VECs) to establish models in vivo and in vitro, then the protective effects of Dex on the vascular endothelial barrier function of sepsis were observed, meanwhile, related mechanisms on regulating mitochondrial fission were further studied. The results showed that Dex could significantly reduce the permeability of pulmonary veins and mesenteric vessels, increase the expression of intercellular junction proteins, enhance the transendothelial electrical resistance and decrease the transmittance of VECs, accordingly protected organ functions and prolonged survival time in septic rats. Besides, the mitochondria of VECs were excessive division after sepsis, while Dex could significantly inhibit the mitochondrial fission and protect mitochondrial function by restoring mitochondrial morphology of VECs. Furthermore, the results showed that ER-MITO contact sites of VECs were notably increased after sepsis. Nevertheless, Dex reduced ER-MITO contact sites by regulating the polymerization of actin via α2 receptors. The results also found that Dex could induce the phosphorylation of the dynamin-related protein 1 through down-regulating extracellular signal-regulated kinase1/2, thus playing a role in the regulation of mitochondrial division. In conclusion, Dex has a protective effect on the vascular endothelial barrier function of septic rats. The mechanism is mainly related to the regulation of Drp1 phosphorylation of VECs, inhibition of mitochondrial division by ER-MITO contacts, and protection of mitochondrial function.
Keywords: Drp1; ER-MITO contact; dexmedetomidine; sepsis; vascular endothelial barrier function.
Copyright © 2021 She, Zhu, Deng, Kuang, Fang, Zhang, Duan, Ye, Zhang, Liu, Hu and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

