Development and In Vivo Characterization of Probiotic Lysate-Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing
- PMID: 33778064
- PMCID: PMC7979291
- DOI: 10.1155/2020/8868618
Development and In Vivo Characterization of Probiotic Lysate-Treated Chitosan Nanogel as a Novel Biocompatible Formulation for Wound Healing
Abstract
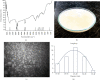
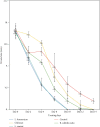
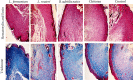
Wound healing is a physiological reaction to tissue injuries which plays a crucial role in replacing the destroyed tissues. Probiotics produce valuable compounds that possess antibacterial and anti-inflammatory activities, immunomodulatory effects, and angiogenesis traits leading to the promotion of wound healing. Chitosan nanostructures have versatile properties making them quickly produced into gels, scaffolds, nanoparticles, beads, and sponge structures that can be incorporated into wound healing processes. In the current study, three formulations from nanogel consisting of probiotic supernatant (Lactobacillus reuteri, Lactobacillus fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels were prepared from the culture of corresponding cultures. The chitosan nanogels were previously characterized by Zetasizer, FTIR, and TEM. The prepared formulations' effectiveness and dressing activity were assessed by evaluating wound closure and histological trials in Sprague-Dawley rats. The results indicated that all probiotic lysate formulations have advantages over the wound healing process. However, Bacillus subtilis natto has a better wound healing quality, which is well known in pathology examination. The favorable effects of probiotic lysate nanogels, including the reasonable wound closing rate, good wound appearance, and satisfactory histological observation via in vivo examination, suggest it as a promising nominee for wound healing purposes.
Copyright © 2020 Yousef Ashoori et al.
Conflict of interest statement
The authors declare that there is not any conflict of interest.
Figures

References
-
- Mousavi S. M., Zarei M., Hashemi S. A., et al. Asymmetric membranes: a potential scaffold for wound healing applications. Symmetry. 2020;12(7)
-
- Nussbaum S. R., Carter M. J., Fife C. E., et al. An economic evaluation of the impact, cost, and medicare policy implications of chronic nonhealing wounds. Value in Health. 2018;21(1):27–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

