Association of Matrix Metalloproteinase-2 mRNA Expression with Subtypes of Pediatric Cholesteatoma
- PMID: 33778077
- PMCID: PMC7972836
- DOI: 10.1155/2021/6644897
Association of Matrix Metalloproteinase-2 mRNA Expression with Subtypes of Pediatric Cholesteatoma
Abstract
Objective: Cholesteatoma is a clinically heterogeneous disease, with some patients showing spontaneous regression, while others experiencing an aggressive, lethal disease. Cholesteatoma in children can be divided into two types: congenital and acquired. Identifying good prognostic markers is needed to help select patients who will require immediate surgical intervention. Matrix metalloproteinase-2 (MMP2) was previously reported to play an important role in cholesteatoma progression, by promoting bone destruction and keratinocyte infiltration. Herein, we analyzed MMP2 mRNA expression level in cholesteatoma using RNA-in situ hybridization in formalin-fixed, paraffin-embedded (FFPE) tissue samples.
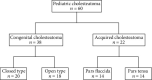
Methods: Sixty patients with cholesteatoma under 15 years old, who underwent their primary surgery at Aichi Medical University's Otolaryngology Department, were analyzed for MMP2 expression level, using RNA-in situ hybridization.
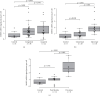
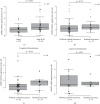
Results: There were no significant differences in MMP2 mRNA expression level between congenital cholesteatoma and acquired cholesteatomas. In congenital cholesteatoma, higher MMP2 signals were observed in the open type than in the closed type (p < 0.001). In acquired cholesteatoma, higher MMP2 signals were observed in the pars tensa than in the pars flaccida (p < 0.001). MMP2 mRNA expression level was almost exclusively found in the fibroblasts or in the inflammatory cells in the stroma, but not in the epithelium.
Conclusion: Our study reveals that MMP2 mRNA expression level is strongly associated with the subtypes of cholesteatoma. The findings suggest that the level of expression of MMP2 mRNA may be related to the pathogenesis and aggressive features of cholesteatoma.
Copyright © 2021 Taichi Kan et al.
Conflict of interest statement
The authors have no significant relationships with or financial interests in any commercial companies pertaining to this article.
Figures


References
-
- Maniu A., Harabagiu O., Perde Schrepler M., Catana A., Fanuta B., Mogoanta C. A. Molecular biology of cholesteatoma. Romanian Journal of Morphology and Embryology. 2014;55(1):7–13. - PubMed
-
- Michaels L. An epidermoid formation in the developing middle ear: possible source of cholesteatoma. The Journal of Otolaryngology. 1986;15(3):169–174. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

