Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner
- PMID: 33778186
- PMCID: PMC7960683
- DOI: 10.1016/j.bioactmat.2021.03.003
Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner
Abstract
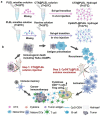
Although neoantigen-based cancer vaccines show great potential in cancer immunotherapy due to their ability to induce effective and long-lasting anti-tumor immunity, their development is hindered by the limitations of neoantigens identification, low immunogenicity, and weak immune response. Cyclophosphamide (CTX) not only directly kills tumors but also causes immunogenic cell death, providing a promising source of antigens for cancer vaccines. Herein, a combined immunotherapy strategy based on temperature-sensitive PLEL hydrogel is designed. First, CTX-loaded hydrogel is injected intratumorally into CT26 bearing mice to prime anti-tumor immunity, and then 3 days later, PLEL hydrogels loaded with CpG and tumor lysates are subcutaneously injected into both groins to further promote anti-tumor immune responses. The results confirm that this combined strategy reduces the toxicity of CTX, and produces the cytotoxic T lymphocyte response to effectively inhibit tumor growth, prolong survival, and significantly improve the tumor cure rate. Moreover, a long-lasting immune memory response is observed in the mice. About 90% of the cured mice survive for at least 60 days after being re-inoculated with tumors, and the distant tumor growth is also well inhibited. Hence, this PLEL-based combination therapy may provide a promising reference for the clinical promotion of chemotherapy combined with cancer vaccines.
Keywords: Cancer vaccine; Cyclophosphamide; Immunogenic cell death; Immunotherapy; Thermo-responsive hydrogels.
© 2021 The Authors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
Intravesical chemotherapy synergize with an immune adjuvant by a thermo-sensitive hydrogel system for bladder cancer.Bioact Mater. 2023 Aug 22;31:315-332. doi: 10.1016/j.bioactmat.2023.08.013. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37663619 Free PMC article.
-
Hydrogel/nanoadjuvant-mediated combined cell vaccines for cancer immunotherapy.Acta Biomater. 2021 Oct 1;133:257-267. doi: 10.1016/j.actbio.2021.08.014. Epub 2021 Aug 15. Acta Biomater. 2021. PMID: 34407475
-
Improvement of anti-tumor immunity of fibroblast activation protein α based vaccines by combination with cyclophosphamide in a murine model of breast cancer.Cell Immunol. 2016 Dec;310:89-98. doi: 10.1016/j.cellimm.2016.08.006. Epub 2016 Aug 16. Cell Immunol. 2016. PMID: 27545090
-
Neoantigen vaccine: an emerging tumor immunotherapy.Mol Cancer. 2019 Aug 23;18(1):128. doi: 10.1186/s12943-019-1055-6. Mol Cancer. 2019. PMID: 31443694 Free PMC article. Review.
-
The present status and future prospects of peptide-based cancer vaccines.Int Immunol. 2016 Jul;28(7):319-28. doi: 10.1093/intimm/dxw027. Epub 2016 May 28. Int Immunol. 2016. PMID: 27235694 Review.
Cited by
-
Nanostructured Biomaterials in 3D Tumor Tissue Engineering Scaffolds: Regenerative Medicine and Immunotherapies.Int J Mol Sci. 2024 May 16;25(10):5414. doi: 10.3390/ijms25105414. Int J Mol Sci. 2024. PMID: 38791452 Free PMC article. Review.
-
Aging-induced immune microenvironment remodeling fosters melanoma in male mice via γδ17-Neutrophil-CD8 axis.Nat Commun. 2024 Dec 30;15(1):10860. doi: 10.1038/s41467-024-55164-3. Nat Commun. 2024. PMID: 39738047 Free PMC article.
-
Regulated extravascular microenvironment via reversible thermosensitive hydrogel for inhibiting calcium influx and vasospasm.Bioact Mater. 2022 Sep 15;21:422-435. doi: 10.1016/j.bioactmat.2022.08.024. eCollection 2023 Mar. Bioact Mater. 2022. PMID: 36185746 Free PMC article.
-
Gel/hydrogel-based in situ biomaterial platforms for cancer postoperative treatment and recovery.Exploration (Beijing). 2023 May 31;3(5):20220173. doi: 10.1002/EXP.20220173. eCollection 2023 Oct. Exploration (Beijing). 2023. PMID: 37933278 Free PMC article. Review.
-
Injectable and thermosensitive hydrogels mediating a universal macromolecular contrast agent with radiopacity for noninvasive imaging of deep tissues.Bioact Mater. 2021 May 23;6(12):4717-4728. doi: 10.1016/j.bioactmat.2021.05.013. eCollection 2021 Dec. Bioact Mater. 2021. PMID: 34136722 Free PMC article.
References
-
- da Silva J.L., Dos Santos A.L.S., Nunes N.C.C., de Moraes Lino da Silva F., Ferreira C.G.M., de Melo A.C. Cancer immunotherapy: the art of targeting the tumor immune microenvironment, Cancer Chemother. Pharmacol. 2019;84(2):227–240. - PubMed
-
- Zhou Z.F., Lin H., Li C., Wu Z.M. Recent progress of fully synthetic carbohydrate-based vaccine using TLR agonist as build-in adjuvant. Chin. Chem. Lett. 2018;29(1):19–26.
-
- Wei C., Dong X., Liang J., Zhang Y., Zhu D.W., Kong D.L., Lv F. Real-time imaging tracking of a dual fluorescent vaccine delivery system based on ovalbumin loaded zinc phthalocyanine-incorporated copolymer nanoparticles. J. Biomed. Nanotechnol. 2019;15(1):100–112. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

