An in situ-forming polyzwitterion hydrogel: Towards vitreous substitute application
- PMID: 33778190
- PMCID: PMC7960944
- DOI: 10.1016/j.bioactmat.2021.02.029
An in situ-forming polyzwitterion hydrogel: Towards vitreous substitute application
Abstract
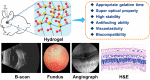
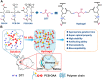
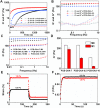
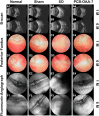
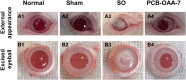
Development of a biostable and biosafe vitreous substitute is highly desirable, but remains a grand challenge. Herein, we propose a novel strategy for constructing a readily administered vitreous substitute based on a thiol-acrylate clickable polyzwitterion macromonomer. A biocompatible multivinyl polycarboxybetaine (PCB-OAA) macromonomer is designed and synthesized, and mixed with dithiothreitol (DTT) via a Michael addition reaction to form a hydrogel in vitreous cavity. This resultant PCB-OAA hydrogel exhibits controllable gelation time, super anti-fouling ability against proteins and cells, excellent biocompatibility, and approximate key parameters to human vitreous body including equilibrium water content, density, optical properties, modulus. Remarkably, outperforming clinically used silicone oil in biocompatibility, this rapidly formed hydrogel in the vitreous cavity of rabbit eyes remains stable in vitreous cavity, showing an appealing ability to prevent significantly inflammatory response, fibrosis and complications such as raised intraocular pressure (IOP), and cataract formation. This zwitterionic polymer hydrogel holds great potential as a vitreous substitute.
Keywords: Anti-fouling; Hydrogel; Vitreous substitute; Zwitterion.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Kleinberg T.T., Tzekov R.T., Stein L., Ravi N., Kaushal S. Vitreous substitutes: a comprehensive review. Surv. Ophthalmol. 2011;56(4):300–323. - PubMed
-
- Baino F. Towards an ideal biomaterial for vitreous replacement: historical overview and future trends. Acta Biomater. 2011;7(3):921–935. - PubMed
-
- Recchia F.M., Ruby A.J., Carvalho Recchia C.A. Pars plana vitrectomy with removal of the internal limiting membrane in the treatment of persistent diabetic macular edema. Am. J. Ophthalmol. 2005;139(3):447–454. - PubMed
-
- Kim R., Baumal C. Anterior segment complications related to vitreous substitutes. Ophthalmol. Clin. North. Am. 2004;17(4):569–576. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

