Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration
- PMID: 33778191
- PMCID: PMC7960680
- DOI: 10.1016/j.bioactmat.2021.02.024
Effect of cyclic mechanical loading on immunoinflammatory microenvironment in biofabricating hydroxyapatite scaffold for bone regeneration
Abstract
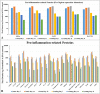
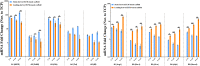
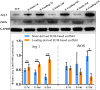
It has been proven that the mechanical microenvironment can impact the differentiation of mesenchymal stem cells (MSCs). However, the effect of mechanical stimuli in biofabricating hydroxyapatite scaffolds on the inflammatory response of MSCs remains unclear. This study aimed to investigate the effect of mechanical loading on the inflammatory response of MSCs seeded on scaffolds. Cyclic mechanical loading was applied to biofabricate the cell-scaffold composite for 15 min/day over 7, 14, or 21 days. At the predetermined time points, culture supernatant was collected for inflammatory mediator detection, and gene expression was analyzed by qRT-PCR. The results showed that the expression of inflammatory mediators (IL1B and IL8) was downregulated (p < 0.05) and the expression of ALP (p < 0.01) and COL1A1 (p < 0.05) was upregulated under mechanical loading. The cell-scaffold composites biofabricated with or without mechanical loading were freeze-dried to prepare extracellular matrix-based scaffolds (ECM-based scaffolds). Murine macrophages were seeded on the ECM-based scaffolds to evaluate their polarization. The ECM-based scaffolds that were biofabricated with mechanical loading before freeze-drying enhanced the expression of M2 polarization-related biomarkers (Arginase 1 and Mrc1, p < 0.05) of macrophages in vitro and increased bone volume/total volume ratio in vivo. Overall, these findings demonstrated that mechanical loading could dually modulate the inflammatory responses and osteogenic differentiation of MSCs. Besides, the ECM-based scaffolds that were biofabricated with mechanical loading before freeze-drying facilitated the M2 polarization of macrophages in vitro and bone regeneration in vivo. Mechanical loading may be a promising biofabrication strategy for bone biomaterials.
Keywords: Biofabrication; Bioreactor; Bone biomaterials; Inflammatory microenvironment; Macrophage polarization.
© 2021 The Authors.
Figures







Similar articles
-
Effect of different hydroxyapatite incorporation methods on the structural and biological properties of porous collagen scaffolds for bone repair.J Anat. 2015 Dec;227(6):732-45. doi: 10.1111/joa.12262. Epub 2014 Nov 20. J Anat. 2015. PMID: 25409684 Free PMC article.
-
Combination of Human Amniotic Fluid Derived-Mesenchymal Stem Cells and Nano-hydroxyapatite Scaffold Enhances Bone Regeneration.Open Access Maced J Med Sci. 2019 Sep 14;7(17):2739-2750. doi: 10.3889/oamjms.2019.730. eCollection 2019 Sep 15. Open Access Maced J Med Sci. 2019. PMID: 31844430 Free PMC article.
-
In vitro response of the bone marrow-derived mesenchymal stem cells seeded in a type-I collagen-glycosaminoglycan scaffold for skin wound repair under the mechanical loading condition.Mol Cell Biomech. 2009 Dec;6(4):217-27. Mol Cell Biomech. 2009. PMID: 19899445
-
Three-Dimensional Mechanical Loading Modulates the Osteogenic Response of Mesenchymal Stem Cells to Tumor-Derived Soluble Signals.Tissue Eng Part A. 2016 Aug;22(15-16):1006-15. doi: 10.1089/ten.TEA.2016.0153. Epub 2016 Aug 1. Tissue Eng Part A. 2016. PMID: 27401765 Free PMC article.
-
Inflammation Responses to Bone Scaffolds under Mechanical Stimuli in Bone Regeneration.J Funct Biomater. 2023 Mar 21;14(3):169. doi: 10.3390/jfb14030169. J Funct Biomater. 2023. PMID: 36976093 Free PMC article. Review.
Cited by
-
MSCs-Derived Decellularised Matrix: Cellular Responses and Regenerative Dentistry.Int Dent J. 2024 Jun;74(3):403-417. doi: 10.1016/j.identj.2024.02.011. Epub 2024 Mar 16. Int Dent J. 2024. PMID: 38494389 Free PMC article. Review.
-
Risk factors for proximal radial abnormalities in children with untreated chronic Monteggia fractures: a review of 142 cases.J Orthop Traumatol. 2024 Nov 29;25(1):60. doi: 10.1186/s10195-024-00793-z. J Orthop Traumatol. 2024. PMID: 39614016 Free PMC article.
-
Sources, Characteristics, and Therapeutic Applications of Mesenchymal Cells in Tissue Engineering.Tissue Eng Regen Med. 2022 Apr;19(2):325-361. doi: 10.1007/s13770-021-00417-1. Epub 2022 Jan 29. Tissue Eng Regen Med. 2022. PMID: 35092596 Free PMC article. Review.
-
Resorbable Biomaterials Used for 3D Scaffolds in Tissue Engineering: A Review.Materials (Basel). 2023 Jun 8;16(12):4267. doi: 10.3390/ma16124267. Materials (Basel). 2023. PMID: 37374451 Free PMC article. Review.
-
Advances in biomaterials for oral-maxillofacial bone regeneration: spotlight on periodontal and alveolar bone strategies.Regen Biomater. 2024 Jul 4;11:rbae078. doi: 10.1093/rb/rbae078. eCollection 2024. Regen Biomater. 2024. PMID: 39055303 Free PMC article. Review.
References
-
- Parikh S.N. Bone graft substitutes: past, present, future. J. Postgrad. Med. 2002;48:142–148. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

