Delivery of pOXR1 through an injectable liposomal nanoparticle enhances spinal cord injury regeneration by alleviating oxidative stress
- PMID: 33778197
- PMCID: PMC7970014
- DOI: 10.1016/j.bioactmat.2021.03.001
Delivery of pOXR1 through an injectable liposomal nanoparticle enhances spinal cord injury regeneration by alleviating oxidative stress
Abstract
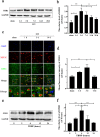
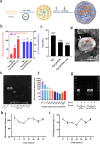
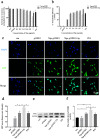
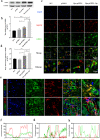
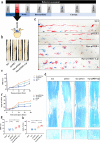
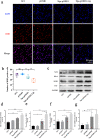
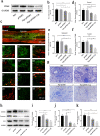
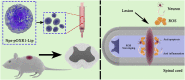
Oxidation resistance 1 (OXR1) is regarded as a critical regulator of cellular homeostasis in response to oxidative stress. However, the role of OXR1 in the neuronal response to spinal cord injury (SCI) remains undefined. On the other hand, gene therapy for SCI has shown limited success to date due in part to the poor utility of conventional gene vectors. In this study, we evaluated the function of OXR1 in SCI and developed an available carrier for delivering the OXR1 plasmid (pOXR1). We found that OXR1 expression is remarkably increased after SCI and that this regulation is protective after SCI. Meanwhile, we assembled cationic nanoparticles with vitamin E succinate-grafted ε-polylysine (VES-g-PLL) (Nps). The pOXR1 was precompressed with Nps and then encapsulated into cationic liposomes. The particle size of pOXR1 was compressed to 58 nm, which suggests that pOXR1 can be encapsulated inside liposomes with high encapsulation efficiency and stability to enhance the transfection efficiency. The agarose gel results indicated that Nps-pOXR1-Lip eliminated the degradation of DNA by DNase I and maintained its activity, and the cytotoxicity results indicated that pOXR1 was successfully transported into cells and exhibited lower cytotoxicity. Finally, Nps-pOXR1-Lip promoted functional recovery by alleviating neuronal apoptosis, attenuating oxidative stress and inhibiting inflammation. Therefore, our study provides considerable evidence that OXR1 is a beneficial factor in resistance to SCI and that Nps-Lip-pOXR1 exerts therapeutic effects in acute traumatic SCI.
Keywords: DNA condensed agent; Gene therapy; OXR1; Oxidative stress; Spinal cord injury.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures


Similar articles
-
Overexpression of HIPK2 attenuates spinal cord injury in rats by modulating apoptosis, oxidative stress, and inflammation.Biomed Pharmacother. 2018 Jul;103:127-134. doi: 10.1016/j.biopha.2018.03.117. Epub 2018 Apr 24. Biomed Pharmacother. 2018. PMID: 29649627
-
Delivering Oxidation Resistance-1 (OXR1) to Mouse Kidney by Genetic Modified Mesenchymal Stem Cells Exhibited Enhanced Protection against Nephrotoxic Serum Induced Renal Injury and Lupus Nephritis.J Stem Cell Res Ther. 2014 Sep 10;4(9):231. doi: 10.4172/2157-7633.1000231. J Stem Cell Res Ther. 2014. PMID: 25995969 Free PMC article.
-
Apolipoprotein E Deficiency Exacerbates Spinal Cord Injury in Mice: Inflammatory Response and Oxidative Stress Mediated by NF-κB Signaling Pathway.Front Cell Neurosci. 2018 May 23;12:142. doi: 10.3389/fncel.2018.00142. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29875635 Free PMC article.
-
Preventing Neurodegeneration by Controlling Oxidative Stress: The Role of OXR1.Front Neurosci. 2020 Dec 15;14:611904. doi: 10.3389/fnins.2020.611904. eCollection 2020. Front Neurosci. 2020. PMID: 33384581 Free PMC article. Review.
-
Oxidative stress in spinal cord injury and antioxidant-based intervention.Spinal Cord. 2012 Apr;50(4):264-74. doi: 10.1038/sc.2011.111. Epub 2011 Oct 11. Spinal Cord. 2012. PMID: 21987065 Review.
Cited by
-
Controlled release of MIF siRNA and GDNF protein from a photocurable scaffold efficiently repairs spinal cord injury.MedComm (2020). 2025 Feb 17;6(3):e70099. doi: 10.1002/mco2.70099. eCollection 2025 Mar. MedComm (2020). 2025. PMID: 39968499 Free PMC article.
-
Inhibiting tau protein improves the recovery of spinal cord injury in rats by alleviating neuroinflammation and oxidative stress.Neural Regen Res. 2023 Aug;18(8):1834-1840. doi: 10.4103/1673-5374.363183. Neural Regen Res. 2023. PMID: 36751813 Free PMC article.
-
Basic fibroblast growth factor-loaded methacrylate gelatin hydrogel microspheres for spinal nerve regeneration.Smart Med. 2023 Mar 28;2(2):e20220038. doi: 10.1002/SMMD.20220038. eCollection 2023 May. Smart Med. 2023. PMID: 39188281 Free PMC article.
-
The Role of Vitamins in Spinal Cord Injury: Mechanisms and Benefits.Oxid Med Cell Longev. 2024 Jun 20;2024:4293391. doi: 10.1155/2024/4293391. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 38938696 Free PMC article. Review.
-
Functional resveratrol-biodegradable manganese doped silica nanoparticles for the spinal cord injury treatment.Mater Today Bio. 2021 Dec 4;13:100177. doi: 10.1016/j.mtbio.2021.100177. eCollection 2022 Jan. Mater Today Bio. 2021. PMID: 34938991 Free PMC article.
References
-
- Rao S., Lin Y., Du Y., He L., Huang G., Chen B., Chen T. Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J. Mater. Chem. B. 2019;7(16):2648–2656. - PubMed
-
- Suzuki K., Elegheert J., Song I., Sasakura H., Senkov O., Matsuda K., Kakegawa W., Clayton A., Chang V., Ferrer-Ferrer M., Miura E., Kaushik R., Ikeno M., Morioka Y., Takeuchi Y., Shimada T., Otsuka S., Stoyanov S., Watanabe M., Takeuchi K., Dityatev A., Aricescu A., Yuzaki M. A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science. 2020;369(6507) - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

