Ion therapy of pulmonary fibrosis by inhalation of ionic solution derived from silicate bioceramics
- PMID: 33778199
- PMCID: PMC7966967
- DOI: 10.1016/j.bioactmat.2021.02.013
Ion therapy of pulmonary fibrosis by inhalation of ionic solution derived from silicate bioceramics
Abstract
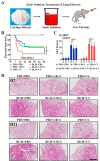
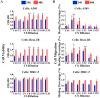
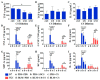
Pulmonary fibrosis (PF) is a chronic and progressively fatal disease, but clinically available therapeutic drugs are limited due to efficacy and side effects. The possible mechanism of pulmonary fibrosis includes the damage of alveolar epithelial cells II (AEC2), and activation of immune cells such as macrophages. The ions released from bioceramics have shown the activity in stimulating soft tissue derived cells such as fibroblasts, endothelia cells and epithelia cells, and regulating macrophage polarization. Therefore, this study proposes an "ion therapy" approach based on the active ions of bioceramic materials, and investigates the therapeutic effect of bioactive ions derived from calcium silicate (CS) bioceramics on mouse models of pulmonary fibrosis. We demonstrate that silicate ions significantly reduce pulmonary fibrosis by simultaneously regulating the functions of AEC2 and macrophages. This result suggests potential clinical applications of ion therapy for lung fibrosis.
Keywords: Alveolar epithelial cells II; Lung fibrosis; Macrophages; Silicate bioceramics.
© 2021 The Authors.
Conflict of interest statement
There are no conflicts of interest in this work.
Figures





Similar articles
-
A new method for treating chronic pancreatitis and preventing fibrosis using bioactive calcium silicate ion solution.J Mater Chem B. 2023 Oct 6;11(38):9163-9178. doi: 10.1039/d3tb01287e. J Mater Chem B. 2023. PMID: 37642526
-
Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration.Acta Biomater. 2018 Jan 15;66:81-92. doi: 10.1016/j.actbio.2017.08.044. Epub 2017 Aug 30. Acta Biomater. 2018. PMID: 28864248
-
Novel Co-akermanite (Ca2CoSi2O7) bioceramics with the activity to stimulate osteogenesis and angiogenesis.J Mater Chem B. 2015 Sep 7;3(33):6773-6782. doi: 10.1039/c5tb01244a. Epub 2015 Jul 29. J Mater Chem B. 2015. PMID: 32262470
-
Silicate bioceramics: from soft tissue regeneration to tumor therapy.J Mater Chem B. 2019 Sep 18;7(36):5449-5460. doi: 10.1039/c9tb01467e. J Mater Chem B. 2019. PMID: 31482927 Review.
-
The role of macrophage polarization and cellular crosstalk in the pulmonary fibrotic microenvironment: a review.Cell Commun Signal. 2024 Mar 9;22(1):172. doi: 10.1186/s12964-024-01557-2. Cell Commun Signal. 2024. PMID: 38461312 Free PMC article. Review.
Cited by
-
Silicate ions as soluble form of bioactive ceramics alleviate aortic aneurysm and dissection.Bioact Mater. 2022 Jul 16;25:716-731. doi: 10.1016/j.bioactmat.2022.07.005. eCollection 2023 Jul. Bioact Mater. 2022. PMID: 37056259 Free PMC article.
-
Oral Delivery of Bioactive Glass-Loaded Core-Shell Hydrogel Microspheres for Effective Treatment of Inflammatory Bowel Disease.Adv Sci (Weinh). 2023 Jun;10(18):e2207418. doi: 10.1002/advs.202207418. Epub 2023 Apr 24. Adv Sci (Weinh). 2023. PMID: 37092589 Free PMC article.
-
Bioceramic scaffolds with triply periodic minimal surface architectures guide early-stage bone regeneration.Bioact Mater. 2023 Feb 17;25:374-386. doi: 10.1016/j.bioactmat.2023.02.012. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36865987 Free PMC article.
-
Silicate-based therapy for inflammatory dilated cardiomyopathy by inhibiting the vicious cycle of immune inflammation via FOXO signaling.Sci Adv. 2025 Apr 11;11(15):eadr7208. doi: 10.1126/sciadv.adr7208. Epub 2025 Apr 9. Sci Adv. 2025. PMID: 40203118 Free PMC article.
-
A New Strategy to Inhibit Scar Formation by Accelerating Normal Healing Using Silicate Bioactive Materials.Adv Sci (Weinh). 2024 Nov;11(43):e2407718. doi: 10.1002/advs.202407718. Epub 2024 Sep 28. Adv Sci (Weinh). 2024. PMID: 39340818 Free PMC article.
References
-
- Adachi T., Chong J.-M., Nakajima N., Sano M., Yamazaki J., Miyamoto I., Nishioka H., Akita H., Sato Y., Kataoka M., Katano H., Tobiume M., Sekizuka T., Itokawa K., Kuroda M., Suzuki T. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerg. Infect. Dis. 2020;26(9):2157–2161. - PMC - PubMed
-
- Bao L., Deng W., Huang B., Gao H., Liu J., Ren L., Wei Q., Yu P., Xu Y., Qi F., Qu Y., Li F., Lv Q., Wang W., Xue J., Gong S., Liu M., Wang G., Wang S., Song Z., Zhao L., Liu P., Zhao L., Ye F., Wang H., Zhou W., Zhu N., Zhen W., Yu H., Zhang X., Guo L., Chen L., Wang C., Wang Y., Wang X., Xiao Y., Sun Q., Liu H., Zhu F., Ma C., Yan L., Yang M., Han J., Xu W., Tan W., Peng X., Jin Q., Wu G., Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583(7818):830–+. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

