l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration
- PMID: 33778205
- PMCID: PMC7970259
- DOI: 10.1016/j.bioactmat.2021.02.035
l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration
Abstract
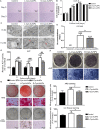
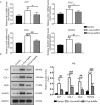
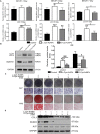
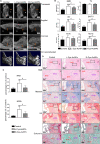
Gold nanoparticles (AuNPs) with surface-anchored molecules present tremendous potential in tissue regeneration. However, little is known about chiral-modified AuNPs. In this study, we successfully prepared L/D-cysteine-anchored AuNPs (L/D-Cys-AuNPs) and studied the effects of chiral-modified AuNPs on osteogenic differentiation and autophagy of human periodontal ligament cells (hPDLCs) and periodontal tissue regeneration. In vitro, more L-Cys-AuNPs than D-Cys-AuNPs tend to internalize in hPDLCs. L-Cys-AuNPs also significantly increased the expression of alkaline phosphatase, collagen type 1, osteocalcin, runt-related transcription factor 2, and microtubule-associated protein light chain 3 II and decreased the expression of sequestosome 1 in hPDLCs compared to the expression levels in the hPDLCs treated by D-Cys-AuNPs. In vivo tests in a rat periodontal-defect model showed that L-Cys-AuNPs had the greatest effect on periodontal-tissue regeneration. The activation of autophagy in L-Cys-AuNP-treated hPDLCs may be responsible for the cell differentiation and tissue regeneration. Therefore, compared to D-Cys-AuNPs, L-Cys-AuNPs show a better performance in cellular internalization, regulation of autophagy, cell osteogenic differentiation, and periodontal tissue regeneration. This demonstrates the immense potential of L-Cys-AuNPs for periodontal regeneration and provides a new insight into chirally modified bioactive nanomaterials.
Keywords: Autophagy; Chirality; Gold nanoparticles; Periodontal; Tissue regeneration.
© 2021 The Authors.
Conflict of interest statement
None.
Figures


References
-
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. - PubMed
-
- Offenbacher S., Jiao Y., Kim S.J., Marchesan J., Moss K.L., Jing L., Divaris K., Bencharit S., Agler C.S., Morelli T., Zhang S., Sun L., Seaman W.T., Cowley D., Barros S.P., Beck J.D., Munz M., Schaefer A.S., North K.E. GWAS for Interleukin-1beta levels in gingival crevicular fluid identifies IL37 variants in periodontal inflammation. Nat. Commun. 2018;9(1):3686. - PMC - PubMed
-
- Seo B.-M., Miura M., Gronthos S., Mark Bartold P., Batouli S., Brahim J., Young M., Gehron Robey P., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364(9429):149–155. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

