Hydrothermal Synthesis of K2Ti6O13 Nanotubes/Nanoparticles: A Photodegradation Study on Methylene Blue and Rhodamine B Dyes
- PMID: 33778239
- PMCID: PMC7992068
- DOI: 10.1021/acsomega.0c02087
Hydrothermal Synthesis of K2Ti6O13 Nanotubes/Nanoparticles: A Photodegradation Study on Methylene Blue and Rhodamine B Dyes
Abstract
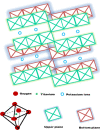
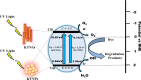
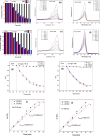
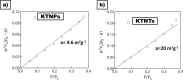
The degradation of methylene blue and rhodamine B dyes using potassium hexatitanate nanoparticles (KTNPs) and potassium hexatitanate nanotubes (KTNTs) synthesized via a hydrothermal method as efficient photocatalysts under UV light irradiation was investigated. The kinetics of degradation was determined for the two different catalysts--KTNPs and KTNTs--by monitoring the optical absorption of the dyes. The as-synthesized KTNPs were found to be spherical in shape with an average particle size of ∼36 ± 1.7 nm, whereas the KTNTs evidenced a tubular hollow structure with ∼7 nm internal diameter and ∼12 nm external diameter, as perused by structural and morphological studies. The larger surface area of KTNTs showed a greater impact on the photodegradation of dyes manifesting their high potential as compared to KTNPs under UV irradiation, and the reusability studies showed more than 90% (KTNTs) and 80% (KTNPs) degradation of the dyes even after the fourth cycle elucidating their stability.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Kim Y.; McCoy L. T.; Lee E.; Lee H.; Saremi R.; Feit C.; Hardin I. R.; Sharma S.; Mani S.; Minko S. Environmentally Sound Textile Dyeing Technology with Nanofibrillated Cellulose. Green Chem. 2017, 19, 4031–4035. 10.1039/c7gc01662j. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

