Selective Extraction of Medium-Chain Carboxylic Acids by Electrodialysis and Phase Separation
- PMID: 33778296
- PMCID: PMC7992139
- DOI: 10.1021/acsomega.1c00397
Selective Extraction of Medium-Chain Carboxylic Acids by Electrodialysis and Phase Separation
Abstract
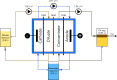
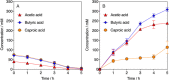
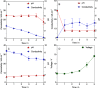
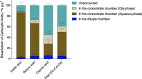
Carboxylic acids obtained via the microbial electrochemical conversion of waste gases containing carbon dioxide (i.e., microbial electrosynthesis) can be used in lieu of nonrenewable building-block chemicals in the manufacture of a variety of products. When targeting valuable medium-chain carboxylic acids such as caproic acid, electricity-driven fermentations can be limited by the accumulation of fermentation products in the culturing media, often resulting in low volumetric productivities and titers due to direct toxicity or inhibition of the biocatalyst. In this study, we tested the effectiveness of a simple electrodialysis system in upconcentrating carboxylic acids from a model solution mimicking the effluent of a microbial electrochemical system producing short- and medium-chain carboxylic acids. Under batch extraction conditions, the electrodialysis scheme enabled the recovery of 60% (mol mol-1) of the total carboxylic acids present in the model fermentation broth. The particular arrangement of conventional monopolar ion exchange membranes and hydraulic recirculation loops allowed the progressive acidification of the extraction solution, enabling phase separation of caproic acid as an immiscible oil with 76% purity.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Direct Medium-Chain Carboxylic Acid Oil Separation from a Bioreactor by an Electrodialysis/Phase Separation Cell.Environ Sci Technol. 2021 Jan 5;55(1):634-644. doi: 10.1021/acs.est.0c04939. Epub 2020 Dec 21. Environ Sci Technol. 2021. PMID: 33347746
-
Upgrading syngas fermentation effluent using Clostridium kluyveri in a continuous fermentation.Biotechnol Biofuels. 2017 Mar 29;10:83. doi: 10.1186/s13068-017-0764-6. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 28367228 Free PMC article.
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Elevated caproic acid production from one-stage anaerobic fermentation of organic waste and its selective recovery by electro-membrane process.Bioresour Technol. 2024 May;399:130647. doi: 10.1016/j.biortech.2024.130647. Epub 2024 Mar 30. Bioresour Technol. 2024. PMID: 38561152
-
Recovery of carboxylic acids produced by fermentation.Biotechnol Adv. 2014 Sep-Oct;32(5):873-904. doi: 10.1016/j.biotechadv.2014.04.002. Epub 2014 Apr 18. Biotechnol Adv. 2014. PMID: 24751382 Review.
Cited by
-
On the Use of Permselectivity to Describe the Selective Transfer of Organic Acids in Electrodialysis.Membranes (Basel). 2023 May 23;13(6):545. doi: 10.3390/membranes13060545. Membranes (Basel). 2023. PMID: 37367749 Free PMC article.
-
Research on the Resource Recovery of Medium-Chain Fatty Acids from Municipal Sludge: Current State and Future Prospects.Microorganisms. 2024 Mar 28;12(4):680. doi: 10.3390/microorganisms12040680. Microorganisms. 2024. PMID: 38674623 Free PMC article. Review.
References
-
- Lee W. S.; Chua A. S. M.; Yeoh H. K.; Ngoh G. C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. 10.1016/j.cej.2013.09.002. - DOI
-
- Cavalcante W. d. A.; Leitão R. C.; Gehring T. A.; Angenent L. T.; Santaella S. T. Anaerobic Fermentation for N-Caproic Acid Production: A Review. Process Biochem. 2017, 54, 106.10.1016/j.procbio.2016.12.024. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

