Abnormal Bone Collagen Cross-Linking in Osteogenesis Imperfecta/Bruck Syndrome Caused by Compound Heterozygous PLOD2 Mutations
- PMID: 33778323
- PMCID: PMC7990156
- DOI: 10.1002/jbm4.10454
Abnormal Bone Collagen Cross-Linking in Osteogenesis Imperfecta/Bruck Syndrome Caused by Compound Heterozygous PLOD2 Mutations
Abstract
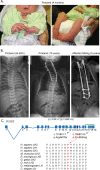
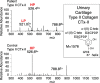
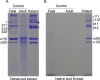
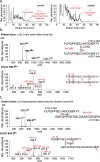
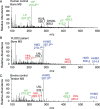
Bruck syndrome (BS) is a congenital disorder characterized by joint flexion contractures, skeletal dysplasia, and increased bone fragility, which overlaps clinically with osteogenesis imperfecta (OI). On a genetic level, BS is caused by biallelic mutations in either FKBP10 or PLOD2. PLOD2 encodes the lysyl hydroxylase 2 (LH2) enzyme, which is responsible for the hydroxylation of cross-linking lysine residues in fibrillar collagen telopeptide domains. This modification enables collagen to form chemically stable (permanent) intermolecular cross-links in the extracellular matrix. Normal bone collagen develops a unique mix of such stable and labile lysyl-oxidase-mediated cross-links, which contribute to bone strength, resistance to microdamage, and crack propagation, as well as the ordered deposition of mineral nanocrystals within the fibrillar collagen matrix. Bone from patients with BS caused by biallelic FKBP10 mutations has been shown to have abnormal collagen cross-linking; however, to date, no direct studies of human bone from BS caused by PLOD2 mutations have been reported. Here the results from a study of a 4-year-old boy with BS caused by compound heterozygous mutations in PLOD2 are discussed. Diminished hydroxylation of type I collagen telopeptide lysines but normal hydroxylation at triple-helical sites was found. Consequently, stable trivalent cross-links were essentially absent. Instead, allysine aldol dimeric cross-links dominated as in normal skin collagen. Furthermore, in contrast to the patient's bone collagen, telopeptide lysines in cartilage type II collagen cross-linked peptides from the patient's urine were normally hydroxylated. These findings shed light on the complex mechanisms that control the unique posttranslational chemistry and cross-linking of bone collagen, and how, when defective, they can cause brittle bones and related connective tissue problems. © 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.
Keywords: BRUCK SYNDROME; COLLAGEN; LYSYL HYDROXYLASE 2; OSTEOGENESIS IMPERFECTA; PLOD2.
© 2020 The Authors. JBMR Plus published by Wiley Periodicals LLC. on behalf of American Society for Bone and Mineral Research.
Figures



References
-
- Nyman JS, Reyes M, Wang X. Effect of ultrastructural changes on the toughness of bone. Micron. 2005;36(7–8):566–82. - PubMed
-
- Mercer DK, Nicol PF, Kimbembe C, Robins SP. Identification, expression, and tissue distribution of the three rat lysyl hydroxylase isoforms. Biochem Biophys Res Commun. 2003;307(4):803–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
