Electrospun biodegradable poly(ε-caprolactone) membranes for annulus fibrosus repair: Long-term material stability and mechanical competence
- PMID: 33778404
- PMCID: PMC7984019
- DOI: 10.1002/jsp2.1130
Electrospun biodegradable poly(ε-caprolactone) membranes for annulus fibrosus repair: Long-term material stability and mechanical competence
Abstract
Background: Electrospun (ES) poly(ɛ-caprolactone) (PCL) is widely used to provide critical mechanical support in tissue engineering and regenerative medicine applications. Therefore, there is a clear need for understanding the change in the mechanical response of the membranes as the material degrades in physiological conditions.
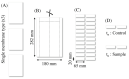
Study design: ES membranes with fiber diameters from 1.6 to 6.7 μm were exposed to in vitro conditions at 37°C in Dulbecco's modified Eagle's medium (DMEM) or dry for up to 6 months.
Methods: During this period, the mechanical properties were assessed using cyclic mechanical loading, and material properties such as crystallinity and ester bond degradation were measured.
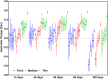
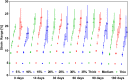
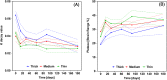
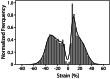
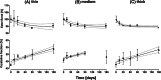
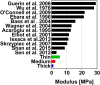
Results: No significant difference was found for any parameters between samples kept dry and in DMEM. The increase in crystallinity was linear with time, while the ester bond degradation showed an inverse logarithmic correlation with time. All samples showed an increase in modulus with exposure time for the first loading cycle. Modulus changes for the consecutive loading cycles showed a nonlinear relationship to the exposure time that depended on membrane type and maximum strain. In addition, the recovered elastic range showed an expected increase with the maximum strain reached. The mechanical response of ES membranes was compared to experimental tensile properties of the human annulus fibrosus tissue and an in silico model of the intervertebral disk. The modulus of the tested membranes was at the lower range of the values found in literature, while the elastically recoverable strain after preconditioning for all membrane types lies within the desired strain range for this application.
Conclusion: The long-term assessment under application-specific conditions allowed to establish the mechanical competence of the electrospun PCL membranes. It can be concluded that with the use of appropriate fixation, the membranes can be used to create a seal on the damaged AF.
Keywords: degradation; electrospinning; intervertebral disk; long‐term; mechanics; poly(ε‐caprolactone); regenerative; repair.
© 2020 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Figures





References
-
- Sell SA, Wolfe PS, Garg K, McCool JM, Rodriguez IA, Bowlin GL. The use of natural polymers in tissue engineering: a focus on electrospun extracellular matrix analogues. Polymers (Basel). 2010;2(4):522‐553.
-
- Cai S, Xu H, Jiang Q, Yang Y. Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: fabrication and mechanism study. Langmuir. 2013;29(7):2311‐2318. - PubMed
-
- Chen ZG, Wang PW, Wei B, Mo XM, Cui FZ. Electrospun collagen‐chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010;6(2):372‐382. - PubMed
-
- Van Lieshout MI, Vaz CM, Rutten MCM, Peters GWM, Baaijens FPT. Electrospinning vs knitting: two scaffolds for tissue engineering of the aortic valve. J Biomater Sci. Polym Ed. 2006;17(1):77‐89. - PubMed
-
- Balguid A, Mol A, van Marion MH, R. A. Bank , Bouten CVC, Baaijens FPT. Tailoring fiber diameter in electrospun poly(ɛ‐Caprolactone) scaffolds for optimal cellular infiltration in cardiovascular tissue engineering. Tissue Eng Part A. 2009;15(2):437‐444. - PubMed
LinkOut - more resources
Full Text Sources

