The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency
- PMID: 33778405
- PMCID: PMC7984018
- DOI: 10.1002/jsp2.1131
The effects of 3D culture on the expansion and maintenance of nucleus pulposus progenitor cell multipotency
Abstract
Introduction: Low back pain (LBP) is a global health concern. Increasing evidence implicates intervertebral disk (IVD) degeneration as a major contributor. In this respect, tissue-specific progenitors may play a crucial role in tissue regeneration, as these cells are perfectly adapted to their niche. Recently, a novel progenitor cell population was described in the nucleus pulposus (NP) that is positive for Tie2 marker. These cells have self-renewal capacity and in vitro multipotency potential. However, extremely low numbers of the NP progenitors limit the feasibility of cell therapy strategies.
Objective: Here, we studied the influence of the culture method and of the microenvironment on the proliferation rate and the differentiation potential of human NP progenitors in vitro.
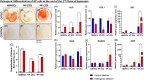
Method: Cells were obtained from human NP tissue from trauma patients. Briefly, the NP tissue cells were cultured in two-dimensional (2D) (monolayer) or three-dimensional (3D) (alginate beads) conditions. After 1 week, cells from 2D or 3D culture were expanded on fibronectin-coated flasks. Subsequently, expanded NP cells were then characterized by cytometry and tri-lineage differentiation, which was analyzed by qPCR and histology. Moreover, experiments using Tie2+ and Tie2- NP cells were also performed.
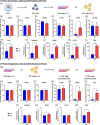
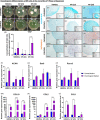
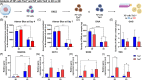
Results: The present study aims to demonstrate that 3D expansion of NP cells better preserves the Tie2+ cell populations and increases the chondrogenic and osteogenic differentiation potential compared to 2D expansion. Moreover, the cell sorting experiments reveal that only Tie2+ cells were able to maintain the pluripotent gene expression if cultured in 3D within alginate beads. Therefore, our results highly suggest that the maintenance of the cell's multipotency is mainly, but not exclusively, due to the higher presence of Tie2+ cells due to 3D culture.
Conclusion: This project not only might have a scientific impact by evaluating the influence of a two-step expansion protocol on the functionality of NP progenitors, but it could also lead to an innovative clinical approach.
Keywords: adipogenesis; alginate bead; angiopoietin‐1 receptor; chondrogenesis; differentiation; flow cytometry; intervertebral disk; microenvironment; nucleus pulposus; osteogenesis; qPCR; three‐dimensional culture; tissue‐specific progenitor cells.
© 2020 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors indicated no potential conflict of interest.
Figures



Similar articles
-
Fluorescence-Activated Cell Sorting Is More Potent to Fish Intervertebral Disk Progenitor Cells Than Magnetic and Beads-Based Methods.Tissue Eng Part C Methods. 2019 Oct;25(10):571-580. doi: 10.1089/ten.TEC.2018.0375. Epub 2019 Sep 9. Tissue Eng Part C Methods. 2019. PMID: 31154900
-
Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells.Stem Cell Res Ther. 2016 May 23;7(1):75. doi: 10.1186/s13287-016-0337-9. Stem Cell Res Ther. 2016. PMID: 27216150 Free PMC article.
-
Spheroid-Like Cultures for Expanding Angiopoietin Receptor-1 (aka. Tie2) Positive Cells from the Human Intervertebral Disc.Int J Mol Sci. 2020 Dec 10;21(24):9423. doi: 10.3390/ijms21249423. Int J Mol Sci. 2020. PMID: 33322051 Free PMC article.
-
Differentiation of Pluripotent Stem Cells into Nucleus Pulposus Progenitor Cells for Intervertebral Disc Regeneration.Curr Stem Cell Res Ther. 2019;14(1):57-64. doi: 10.2174/1574888X13666180918095121. Curr Stem Cell Res Ther. 2019. PMID: 30227822 Review.
-
The nucleus pulposus microenvironment in the intervertebral disc: the fountain of youth?Eur Cell Mater. 2021 Jun 15;41:707-738. doi: 10.22203/eCM.v041a46. Eur Cell Mater. 2021. PMID: 34128534 Review.
Cited by
-
ISSLS PRIZE in Basic Science 2024: superiority of nucleus pulposus cell- versus mesenchymal stromal cell-derived extracellular vesicles in attenuating disc degeneration and alleviating pain.Eur Spine J. 2024 May;33(5):1713-1727. doi: 10.1007/s00586-024-08163-3. Epub 2024 Feb 28. Eur Spine J. 2024. PMID: 38416190
-
Effect of Whole Tissue Culture and Basic Fibroblast Growth Factor on Maintenance of Tie2 Molecule Expression in Human Nucleus Pulposus Cells.Int J Mol Sci. 2021 Apr 29;22(9):4723. doi: 10.3390/ijms22094723. Int J Mol Sci. 2021. PMID: 33946902 Free PMC article.
-
Immuno-Modulatory Effects of Intervertebral Disc Cells.Front Cell Dev Biol. 2022 Jun 29;10:924692. doi: 10.3389/fcell.2022.924692. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35846355 Free PMC article. Review.
-
Recombinant Laminin-511 Fragment (iMatrix-511) Coating Supports Maintenance of Human Nucleus Pulposus Progenitor Cells In Vitro.Int J Mol Sci. 2023 Nov 24;24(23):16713. doi: 10.3390/ijms242316713. Int J Mol Sci. 2023. PMID: 38069038 Free PMC article.
-
Mesenchymal stem cell-derived secretome enhances nucleus pulposus cell metabolism and modulates extracellular matrix gene expression in vitro.Front Bioeng Biotechnol. 2023 Mar 16;11:1152207. doi: 10.3389/fbioe.2023.1152207. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37008028 Free PMC article.
References
-
- Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299(6):656‐664. - PubMed
-
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028‐2037. - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease study 2017. Lancet. 2018;392(10159):1789‐1858. - PMC - PubMed
-
- Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11(4):243‐256. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

