Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons
- PMID: 33778899
- PMCID: PMC8166715
- DOI: 10.1007/s00204-021-03031-1
Functional alterations by a subgroup of neonicotinoid pesticides in human dopaminergic neurons
Abstract
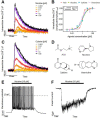
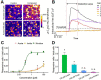
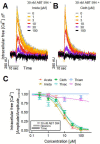
Neonicotinoid pesticides, originally developed to target the insect nervous system, have been reported to interact with human receptors and to activate rodent neurons. Therefore, we evaluated in how far these compounds may trigger signaling in human neurons, and thus, affect the human adult or developing nervous system. We used SH-SY5Y neuroblastoma cells as established model of nicotinic acetylcholine receptor (nAChR) signaling. In parallel, we profiled dopaminergic neurons, generated from LUHMES neuronal precursor cells, as novel system to study nAChR activation in human post-mitotic neurons. Changes of the free intracellular Ca2+ concentration ([Ca2+]i) were used as readout, and key findings were confirmed by patch clamp recordings. Nicotine triggered typical neuronal signaling responses that were blocked by antagonists, such as tubocurarine and mecamylamine. Pharmacological approaches suggested a functional expression of α7 and non-α7 nAChRs on LUHMES cells. In this novel test system, the neonicotinoids acetamiprid, imidacloprid, clothianidin and thiacloprid, but not thiamethoxam and dinotefuran, triggered [Ca2+]i signaling at 10-100 µM. Strong synergy of the active neonicotinoids (at low micromolar concentrations) with the α7 nAChR-positive allosteric modulator PNU-120596 was observed in LUHMES and SH-SY5Y cells, and specific antagonists fully inhibited such signaling. To provide a third line of evidence for neonicotinoid signaling via nAChR, we studied cross-desensitization: pretreatment of LUHMES and SH-SY5Y cells with active neonicotinoids (at 1-10 µM) blunted the signaling response of nicotine. The pesticides (at 3-30 µM) also blunted the response to the non-α7 agonist ABT 594 in LUHMES cells. These data show that human neuronal cells are functionally affected by low micromolar concentrations of several neonicotinoids. An effect of such signals on nervous system development is a toxicological concern.
Keywords: Desensitization; Live-cell calcium imaging; Molecular docking; Neurotoxicity; Nicotine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Molecular Docking Analysis at the Human α7-nAChR and Proliferative and Evoked-Calcium Changes in SH-SY5Y Cells by Imidacloprid and Acetamiprid Insecticides.Neurotox Res. 2024 Feb 20;42(2):16. doi: 10.1007/s12640-024-00697-0. Neurotox Res. 2024. PMID: 38376791
-
Growth and neurite stimulating effects of the neonicotinoid pesticide clothianidin on human neuroblastoma SH-SY5Y cells.Toxicol Appl Pharmacol. 2019 Nov 15;383:114777. doi: 10.1016/j.taap.2019.114777. Epub 2019 Oct 15. Toxicol Appl Pharmacol. 2019. PMID: 31626844
-
Acute effects of the imidacloprid metabolite desnitro-imidacloprid on human nACh receptors relevant for neuronal signaling.Arch Toxicol. 2021 Dec;95(12):3695-3716. doi: 10.1007/s00204-021-03168-z. Epub 2021 Oct 10. Arch Toxicol. 2021. PMID: 34628512 Free PMC article.
-
An Overview on the Effect of Neonicotinoid Insecticides on Mammalian Cholinergic Functions through the Activation of Neuronal Nicotinic Acetylcholine Receptors.Int J Environ Res Public Health. 2020 May 6;17(9):3222. doi: 10.3390/ijerph17093222. Int J Environ Res Public Health. 2020. PMID: 32384754 Free PMC article. Review.
-
Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors.Annu Rev Entomol. 2003;48:339-64. doi: 10.1146/annurev.ento.48.091801.112731. Epub 2002 Jun 4. Annu Rev Entomol. 2003. PMID: 12208819 Review.
Cited by
-
Statement on the toxicological properties and maximum residue levels of acetamiprid and its metabolites.EFSA J. 2024 May 15;22(5):e8759. doi: 10.2903/j.efsa.2024.8759. eCollection 2024 May. EFSA J. 2024. PMID: 38751503 Free PMC article.
-
Multiple Aspects of the Fight against the Red Palm Weevil in an Urban Area: Study Case, San Benedetto del Tronto (Central Italy).Insects. 2023 May 30;14(6):502. doi: 10.3390/insects14060502. Insects. 2023. PMID: 37367318 Free PMC article.
-
Neonicotinoid Residues in Tea Products from China: Contamination Patterns and Implications for Human Exposure.Toxics. 2025 Jun 29;13(7):550. doi: 10.3390/toxics13070550. Toxics. 2025. PMID: 40710995 Free PMC article.
-
Molecular Docking Analysis at the Human α7-nAChR and Proliferative and Evoked-Calcium Changes in SH-SY5Y Cells by Imidacloprid and Acetamiprid Insecticides.Neurotox Res. 2024 Feb 20;42(2):16. doi: 10.1007/s12640-024-00697-0. Neurotox Res. 2024. PMID: 38376791
-
Cytotoxicity induced by three commercial neonicotinoid insecticide formulations in differentiated human neuroblastoma SH-SY5Y cells.Toxicol Res (Camb). 2024 Oct 10;13(5):tfae171. doi: 10.1093/toxres/tfae171. eCollection 2024 Oct. Toxicol Res (Camb). 2024. PMID: 39399211
References
-
- Abou-Donia MB, Goldstein LB, Bullman S, et al. Imidacloprid induces neurobehavioral deficits and increases expression of glial fibrillary acidic protein in the motor cortex and hippocampus in offspring rats following in utero exposure. J Toxicol Environ Health A. 2008;71:119–130. doi: 10.1080/15287390701613140. - DOI - PubMed
-
- Alkondon M, Pereira EFR, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. - DOI - PMC - PubMed
-
- Arias HR, Feuerbach D, Targowska-Duda K, et al. Pharmacological and molecular studies on the interaction of varenicline with different nicotinic acetylcholine receptor subtypes. Potential mechanism underlying partial agonism at human α4β2 and α3β4 subtypes. Biochim Biophys Acta BBA Biomembr. 2015;1848:731–741. doi: 10.1016/j.bbamem.2014.11.003. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

