Impact of slice thickness, pixel size, and CT dose on the performance of automatic contouring algorithms
- PMID: 33779037
- PMCID: PMC8130223
- DOI: 10.1002/acm2.13207
Impact of slice thickness, pixel size, and CT dose on the performance of automatic contouring algorithms
Abstract
Purpose: To investigate the impact of computed tomography (CT) image acquisition and reconstruction parameters, including slice thickness, pixel size, and dose, on automatic contouring algorithms.
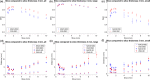
Methods: Eleven scans from patients with head-and-neck cancer were reconstructed with varying slice thicknesses and pixel sizes. CT dose was varied by adding noise using low-dose simulation software. The impact of these imaging parameters on two in-house auto-contouring algorithms, one convolutional neural network (CNN)-based and one multiatlas-based system (MACS) was investigated for 183 reconstructed scans. For each algorithm, auto-contours for organs-at-risk were compared with auto-contours from scans with 3 mm slice thickness, 0.977 mm pixel size, and 100% CT dose using Dice similarity coefficient (DSC), Hausdorff distance (HD), and mean surface distance (MSD).
Results: Increasing the slice thickness from baseline value of 3 mm gave a progressive reduction in DSC and an increase in HD and MSD on average for all structures. Reducing the CT dose only had a relatively minimal effect on DSC and HD. The rate of change with respect to dose for both auto-contouring methods is approximately 0. Changes in pixel size had a small effect on DSC and HD for CNN-based auto-contouring with differences in DSC being within 0.07. Small structures had larger deviations from the baseline values than large structures for DSC. The relative differences in HD and MSD between the large and small structures were small.
Conclusions: Auto-contours can deviate substantially with changes in CT acquisition and reconstruction parameters, especially slice thickness and pixel size. The CNN was less sensitive to changes in pixel size, and dose levels than the MACS. The results contraindicated more restrictive values for the parameters should be used than a typical imaging protocol for head-and-neck.
Keywords: CT parameters; atlas-based segmentation; auto-contouring; convolutional neural network.
© 2021 The Authors. Journal of Applied Clinical Medical Physics published by Wiley Periodicals LLC on behalf of American Association of Physicists in Medicine.
Conflict of interest statement
Other aspects of the Radiation Planning Assistant project are funded by Varian Medical Systems.
Figures


References
-
- Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra‐ and inter‐observer variability in contouring prostate and seminal vesicles: implications for conformal treatment planning. Radiother Oncol. 1998;47:285–292. - PubMed
-
- Klein EE, Hanley J, Bayouth J, et al. Task Group 142 report: quality assurance of medical accelerators. Med Phys. 2009;36:4197–4212. - PubMed
-
- Teguh DN, Levendag PC, Voet PWJ, et al. Clinical validation of atlas‐based auto‐segmentation of multiple target volumes and normal tissue (swallowing/mastication) structures in the head and neck. Int J Radiat Oncol Biol Phys. 2011;81:950–957. - PubMed
-
- Cardenas CE, Yang J, Anderson BM, Court LE, Brock KB. Advances in auto‐segmentation. Semin Radiat Oncol. 2019;29:185–197. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

