Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality
- PMID: 33779075
- PMCID: PMC8120376
- DOI: 10.1002/ehf2.13297
Full spectrum of clonal haematopoiesis-driver mutations in chronic heart failure and their associations with mortality
Abstract
Aims: Somatic mutations in haematopoietic stem cells can lead to the clonal expansion of mutated blood cells, known as clonal haematopoiesis (CH). Mutations in the most prevalent driver genes DNMT3A and TET2 with a variant allele frequency (VAF) ≥ 2% have been associated with atherosclerosis and chronic heart failure of ischemic origin (CHF). However, the effects of mutations in other driver genes for CH with low VAF (<2%) on CHF are still unknown.
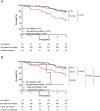
Methods and results: Therefore, we analysed mononuclear bone marrow and blood cells from 399 CHF patients by deep error-corrected targeted sequencing of 56 genes and associated mutations with the long-term mortality in these patients (3.95 years median follow-up). We detected 1113 mutations with a VAF ≥ 0.5% in 347 of 399 patients, and only 13% had no detectable CH. Despite a high prevalence of mutations in the most frequently mutated genes DNMT3A (165 patients) and TET2 (107 patients), mutations in CBL, CEBPA, EZH2, GNB1, PHF6, SMC1A, and SRSF2 were associated with increased death compared with the average death rate of all patients. To avoid confounding effects, we excluded patients with DNMT3A-related, TET2-related, and other clonal haematopoiesis of indeterminate potential (CHIP)-related mutations with a VAF ≥ 2% for further analyses. Kaplan-Meier survival analyses revealed a significantly higher mortality in patients with mutations in either of the seven genes (53 patients), combined as the CH-risk gene set for CHF. Baseline patient characteristics showed no significant differences in any parameter including patient age, confounding diseases, severity of CHF, or blood cell parameters except for a reduced number of platelets in patients with mutations in the risk gene set in comparison with patients without. However, carrying a mutation in any of the risk genes remained significant after multivariate cox regression analysis (hazard ratio, 3.1; 95% confidence interval, 1.8-5.4; P < 0.001), whereas platelet numbers did not.
Conclusions: Somatic mutations with low VAF in a distinct set of genes, namely, in CBL, CEBPA, EZH2, GNB1, PHF6, SMC1A, and SRSF2, are significantly associated with mortality in CHF, independently of the most prevalent CHIP-mutations in DNMT3A and TET2. Mutations in these genes are prevalent in young CHF patients and comprise an independent risk factor for the outcome of CHF, potentially providing a novel tool for risk assessment in CHF.
Keywords: Age; Blood cell mutations; Clonal haematopoiesis; Heart failure.
© 2021 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
All authors declare no competing interests.
Figures



References
-
- Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail 2014; 1: 4–25. - PubMed
-
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017; 377: 111–121. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

