Liver glycogen-induced enhancements in hypoglycemic counterregulation require neuroglucopenia
- PMID: 33779306
- PMCID: PMC8424545
- DOI: 10.1152/ajpendo.00501.2020
Liver glycogen-induced enhancements in hypoglycemic counterregulation require neuroglucopenia
Abstract
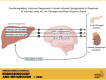
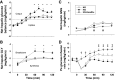
Iatrogenic hypoglycemia is a prominent barrier to achieving optimal glycemic control in patients with diabetes, in part due to dampened counterregulatory hormone responses. It has been demonstrated that elevated liver glycogen content can enhance these hormonal responses through signaling to the brain via afferent nerves, but the role that hypoglycemia in the brain plays in this liver glycogen effect remains unclear. During the first 4 h of each study, the liver glycogen content of dogs was increased by using an intraportal infusion of fructose to stimulate hepatic glucose uptake (HG; n = 13), or glycogen was maintained near fasting levels with a saline infusion (NG; n = 6). After a 2-h control period, during which the fructose/saline infusion was discontinued, insulin was infused intravenously for an additional 2 h to bring about systemic hypoglycemia in all animals, whereas brain euglycemia was maintained in a subset of the HG group by infusing glucose bilaterally into the carotid and vertebral arteries (HG-HeadEu; n = 7). Liver glycogen content was markedly elevated in the two HG groups (43 ± 4, 73 ± 3, and 75 ± 7 mg/g in NG, HG, and HG-HeadEu, respectively). During the hypoglycemic period, arterial plasma glucose levels were indistinguishable between groups (53 ± 2, 52 ± 1, and 51 ± 1 mg/dL, respectively), but jugular vein glucose levels were kept euglycemic (88 ± 5 mg/dL) only in the HG-HeadEu group. Glucagon and epinephrine responses to hypoglycemia were higher in HG compared with NG, whereas despite the increase in liver glycogen, neither increased above basal in HG-HeadEu. These data demonstrate that the enhanced counterregulatory hormone secretion that accompanies increased liver glycogen content requires hypoglycemia in the brain.NEW & NOTEWORTHY It is well known that iatrogenic hypoglycemia is a barrier to optimal glycemic regulation in patients with diabetes. Our data confirm that increasing liver glycogen content 75% above fasting levels enhances hormonal responses to insulin-induced hypoglycemia and demonstrate that this enhanced hormonal response does not occur in the absence of hypoglycemia in the brain. These data demonstrate that information from the liver regarding glycogen availability is integrated in the brain to optimize the counterregulatory response.
Keywords: glucagon; gut amino acid production; hepatic glucose metabolism; type 1 diabetes.
Figures



References
-
- Reinmuth OM, Scheinberg P, Bourne B. Total cerebral blood flow and metabolism: a new method for the repeated serial measurement of total cerbral blood flow using iodoantipyrine (1131) with a report of determination in normal human beings of blood flow, oxygen consumption, glucose utilization and respiratory quotient of the whole brain. Arch Neurol 12: 49–66, 1965. doi: 10.1001/archneur.1965.00460250053007. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

