An open-source device for measuring food intake and operant behavior in rodent home-cages
- PMID: 33779547
- PMCID: PMC8075584
- DOI: 10.7554/eLife.66173
An open-source device for measuring food intake and operant behavior in rodent home-cages
Abstract
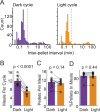
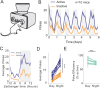
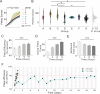
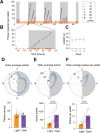
Feeding is critical for survival, and disruption in the mechanisms that govern food intake underlies disorders such as obesity and anorexia nervosa. It is important to understand both food intake and food motivation to reveal mechanisms underlying feeding disorders. Operant behavioral testing can be used to measure the motivational component to feeding, but most food intake monitoring systems do not measure operant behavior. Here, we present a new solution for monitoring both food intake and motivation in rodent home-cages: the Feeding Experimentation Device version 3 (FED3). FED3 measures food intake and operant behavior in rodent home-cages, enabling longitudinal studies of feeding behavior with minimal experimenter intervention. It has a programmable output for synchronizing behavior with optogenetic stimulation or neural recordings. Finally, FED3 design files are open-source and freely available, allowing researchers to modify FED3 to suit their needs.
Keywords: circadian; feeding; mouse; neuroscience; open-source; operant behavior; self-stimulation.
Plain language summary
Obesity and anorexia nervosa are two health conditions related to food intake. Researchers studying these disorders in animal models need to both measure food intake and assess behavioural factors: that is, why animals seek and consume food. Measuring an animal’s food intake is usually done by weighing food containers. However, this can be inaccurate due to the small amount of food that rodents eat. As for studying feeding motivation, this can involve calculating the number of times an animal presses a lever to receive a food pellet. These tests are typically conducted in hour-long sessions in temporary testing cages, called operant boxes. Yet, these tests only measure a brief period of a rodent's life. In addition, it takes rodents time to adjust to these foreign environments, which can introduce stress and may alter their feeding behaviour. To address this, Matikainen-Ankney, Earnest, Ali et al. developed a device for monitoring food intake and feeding behaviours around the clock in rodent home cages with minimal experimenter intervention. This ‘Feeding Experimentation Device’ (FED3) features a pellet dispenser and two ‘nose-poke’ sensors to measure total food intake, as well as motivation for and learning about food rewards. The battery-powered, wire-free device fits in standard home cages, enabling long-term studies of feeding behaviour with minimal intervention from investigators and less stress on the animals. This means researchers can relate data to circadian rhythms and meal patterns, as Matikainen-Ankney did here. Moreover, the device software is open-source so researchers can customise it to suit their experimental needs. It can also be programmed to synchronise with other instruments used in animal experiments, or across labs running the same behavioural tasks for multi-site studies. Used in this way, it could help improve reproducibility and reliability of results from such studies. In summary, Matikainen-Ankney et al. have presented a new practical solution for studying food-related behaviours in mice and rats. Not only could the device be useful to researchers, it may also be suitable to use in educational settings such as teaching labs and classrooms.
Conflict of interest statement
BM, TE, MA, EC, JW, AS, AL, KB, LM, MN, YC, KN, EL, AR, RC, RS, SC, RA, JM, MC, VC, MB, MK, ZA, AK No competing interests declared, FC Director of Open Ephys Production Site
Figures







Comment in
-
Feeding with FED3.Lab Anim (NY). 2021 May;50(5):121. doi: 10.1038/s41684-021-00771-6. Lab Anim (NY). 2021. PMID: 33911250 No abstract available.
References
Publication types
MeSH terms
Grants and funding
- 2017-12-54/Whitehall Foundation
- R01 NS117899/NS/NINDS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R01NS117899/NH/NIH HHS/United States
- DA047127/NH/NIH HHS/United States
- R00 DA038725/DA/NIDA NIH HHS/United States
- ZIA DK075099/ImNIH/Intramural NIH HHS/United States
- 27197/Brain and Behavior Research Foundation
- DA049924/NH/NIH HHS/United States
- F32 DK126355/DK/NIDDK NIH HHS/United States
- DK108742/NH/NIH HHS/United States
- R00DA038725/NH/NIH HHS/United States
- 27416/Brain and Behavior Research Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

