Comparing Long-Acting Antipsychotic Discontinuation Rates Under Ordinary Clinical Circumstances: A Survival Analysis from an Observational, Pragmatic Study
- PMID: 33779944
- PMCID: PMC8219561
- DOI: 10.1007/s40263-021-00809-w
Comparing Long-Acting Antipsychotic Discontinuation Rates Under Ordinary Clinical Circumstances: A Survival Analysis from an Observational, Pragmatic Study
Abstract
Background: Recent guidelines suggested a wider use of long-acting injectable antipsychotics (LAI) than previously, but naturalistic data on the consequences of LAI use in terms of discontinuation rates and associated factors are still sparse, making it hard for clinicians to be informed on plausible treatment courses.
Objective: Our objective was to assess, under real-world clinical circumstances, LAI discontinuation rates over a period of 12 months after a first prescription, reasons for discontinuation, and associated factors.
Methods: The STAR Network 'Depot Study' was a naturalistic, multicentre, observational prospective study that enrolled subjects initiating a LAI without restrictions on diagnosis, clinical severity or setting. Participants from 32 Italian centres were assessed at baseline and at 6 and 12 months of follow-up. Psychopathology, drug attitude and treatment adherence were measured using the Brief Psychiatric Rating Scale, the Drug Attitude Inventory and the Kemp scale, respectively.
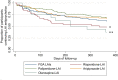
Results: The study followed 394 participants for 12 months. The overall discontinuation rate at 12 months was 39.3% (95% confidence interval [CI] 34.4-44.3), with paliperidone LAI being the least discontinued LAI (33.9%; 95% CI 25.3-43.5) and olanzapine LAI the most discontinued (62.5%; 95% CI 35.4-84.8). The most frequent reason for discontinuation was onset of adverse events (32.9%; 95% CI 25.6-40.9) followed by participant refusal of the medication (20.6%; 95% CI 14.6-27.9). Medication adherence at baseline was negatively associated with discontinuation risk (hazard ratio [HR] 0.853; 95% CI 0.742-0.981; p = 0.026), whereas being prescribed olanzapine LAI was associated with increased discontinuation risk compared with being prescribed paliperidone LAI (HR 2.156; 95% CI 1.003-4.634; p = 0.049).
Conclusions: Clinicians should be aware that LAI discontinuation is a frequent occurrence. LAI choice should be carefully discussed with the patient, taking into account individual characteristics and possible obstacles related to the practicalities of each formulation.
Conflict of interest statement
Giovanni Martinotti has been a consultant and/or a speaker and/or has received research grants from Angelini, Doc Generici, Janssen, Lundbeck, Otsuka, and Pfizer. Federico Bertolini, Giovanni Ostuzzi, Michela Pievani, Andrea Aguglia, Francesco Bartoli, Paola Bortolaso, Camilla Callegari, Mariarita Caroleo, Giuseppe Carrà, Mariangela Corbo, Armando D’Agostino, Pasquale De Fazio, Fabio Magliocco, Edoardo Giuseppe Ostinelli, Marco Piero Piccinelli, Federico Tedeschi and Corrado Barbui have no conflicts of interest that are directly relevant to the content of this article.
Figures

References
-
- Bowtell M, Ratheesh A, McGorry P, Killackey E, O'Donoghue B. Clinical and demographic predictors of continuing remission or relapse following discontinuation of antipsychotic medication after a first episode of psychosis. A systematic review. Schizophr Res. 2018;197:9–18. doi: 10.1016/j.schres.2017.11.010. - DOI - PubMed
-
- Barnes TR, Drake R, Paton C, Cooper SJ, Deakin B, Ferrier IN, et al. Evidence-based guidelines for the pharmacological treatment of schizophrenia: updated recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2020;34(1):3–78. doi: 10.1177/0269881119889296. - DOI - PubMed
-
- Galletly C, Castle D, Dark F, Humberstone V, Jablensky A, Killackey E, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410–472. doi: 10.1177/0004867416641195. - DOI - PubMed
-
- NICE. Psychosis and schizophrenia in adults: prevention and management. Clinical guideline [CG178]. 2014. https://www.nice.org.uk/guidance/cg178. Accessed 1 Sept 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

