Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period
- PMID: 33779986
- PMCID: PMC8092635
- DOI: 10.1002/14651858.CD001689.pub4
Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period
Abstract
Background: Venous thromboembolism (VTE), although rare, is a major cause of maternal mortality and morbidity. Some women are at increased risk of VTE during pregnancy and the early postnatal period (e.g. caesarean section, family history of VTE, or thrombophilia), and so prophylaxis may be considered. As some methods of prophylaxis carry risks of adverse effects, and risk of VTE is often low, benefits of thromboprophylaxis may be outweighed by harms.
Objectives: To assess the effects of thromboprophylaxis during pregnancy and the early postnatal period on the risk of venous thromboembolic disease and adverse effects in women at increased risk of VTE.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (18 October 2019). In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (18 October 2019).
Selection criteria: Randomised trials comparing one method of thromboprophylaxis with placebo or no treatment, or two (or more) methods of thromboprophylaxis.
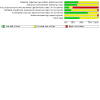
Data collection and analysis: At least two review authors assessed trial eligibility, extracted data, assessed risk of bias, and judged certainty of evidence for selected critical outcomes (using GRADE). We conducted fixed-effect meta-analysis and reported data (all dichotomous) as summary risk ratios (RRs) with 95% confidence intervals (CIs).
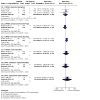
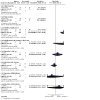
Main results: Twenty-nine trials (involving 3839 women), overall at moderate to high risk of bias were included. Trials were conducted across the antenatal, peripartum and postnatal periods, with most in high-income countries. Interventions included types and regimens of heparin (low molecular weight heparin (LMWH) and unfractionated heparin (UFH)), hydroxyethyl starch (HES), and compression stockings or devices. Data were limited due to a small number of trials in comparisons and/or few or no events reported. All critical outcomes (assessed for comparisons of heparin versus no treatment/placebo, and LMWH versus UFH) were considered to have very low-certainty evidence, downgraded mainly for study limitations and imprecise effect estimates. Maternal death was not reported in most studies. Antenatal (± postnatal) prophylaxis For the primary outcomes symptomatic thromboembolic events pulmonary embolism (PE) and/or deep vein thrombosis (DVT), and the critical outcome of adverse effects sufficient to stop treatment, the evidence was very uncertain. Symptomatic thromboembolic events: - heparin versus no treatment/placebo (RR 0.39; 95% CI 0.08 to 1.98; 4 trials, 476 women; very low-certainty evidence); - LMWH versus UFH (RR 0.47; 95% CI 0.09 to 2.49; 4 trials, 404 women; very low-certainty evidence); Symptomatic PE: - heparin versus no treatment/placebo (RR 0.33; 95% CI 0.02 to 7.14; 3 trials, 187 women; very low-certainty evidence); - LMWH versus UFH (no events; 3 trials, 287 women); Symptomatic DVT: - heparin versus no treatment/placebo (RR 0.33; 95% CI 0.04 to 3.10; 4 trials, 227 women; very low-certainty evidence); - LMWH versus UFH (no events; 3 trials, 287 women); Adverse effects sufficient to stop treatment: - heparin versus no treatment/placebo (RR 0.49; 95% CI 0.05 to 5.31; 1 trial, 139 women; very low-certainty evidence); - LMWH versus UFH (RR 0.07; 95% CI 0.01 to 0.54; 2 trials, 226 women; very low-certainty evidence). Peripartum/postnatal prophylaxis Vaginal or caesarean birth When UFH and no treatment were compared, the effects on symptomatic thromboembolic events (RR 0.16; 95% CI 0.02 to 1.36; 1 trial, 210 women; very low-certainty evidence), symptomatic PE (RR 0.16; 95% CI 0.01 to 3.34; 1 trial, 210 women; very low-certainty evidence), and symptomatic DVT (RR 0.27; 95% CI 0.03 to 2.55; 1 trial, 210 women; very low-certainty evidence) were very uncertain. Maternal death and adverse effects sufficient to stop treatment were not reported. Caesarean birth Symptomatic thromboembolic events: - heparin versus no treatment/placebo (RR 1.30; 95% CI 0.39 to 4.27; 4 trials, 840 women; very low-certainty evidence); - LMWH versus UFH (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; very low-certainty evidence); Symptomatic PE: - heparin versus no treatment/placebo (RR 1.10; 95% CI 0.25 to 4.87; 4 trials, 840 women; very low-certainty evidence); - LMWH versus UFH (no events; 3 trials, 217 women); Symptomatic DVT: - heparin versus no treatment/placebo (RR 1.30; 95% CI 0.24 to 6.94; 5 trials, 1140 women; very low-certainty evidence); LMWH versus UFH (RR 0.33; 95% CI 0.01 to 7.99; 3 trials, 217 women; very low-certainty evidence); Maternal death: - heparin versus placebo (no events, 1 trial, 300 women); Adverse effects sufficient to stop treatment: - heparin versus placebo (no events; 1 trial, 140 women). Postnatal prophylaxis No events were reported for LMWH versus no treatment/placebo for: symptomatic thromboembolic events, symptomatic PE and symptomatic DVT (all 2 trials, 58 women), or maternal death (1 trial, 24 women). Adverse effects sufficient to stop treatment were not reported. We were unable to conduct subgroup analyses due to lack of data. Sensitivity analysis including the nine studies at low risk of bias did not impact overall findings.
Authors' conclusions: The evidence is very uncertain about benefits and harms of VTE thromboprophylaxis in women during pregnancy and the early postnatal period at increased risk of VTE. Further high-quality very large-scale randomised trials are needed to determine effects of currently used treatments in women with different VTE risk factors. As sufficiently large definitive trials are unlikely to be funded, secondary data analyses based on high-quality registry data are important.
Trial registration: ClinicalTrials.gov NCT01321788 NCT00967382 NCT01274637 NCT01068795 NCT02070237 NCT00356434 NCT01588171 NCT00400387 NCT03659708 NCT00225108 NCT00878826 NCT01019655 NCT01828697 NCT04153760.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Judith Gomersall: none known.
Philippa Middleton: none known.
Emily Shepherd: none known.
Figures
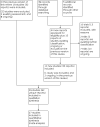




























































































Update of
-
Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period.Cochrane Database Syst Rev. 2014 Feb 11;(2):CD001689. doi: 10.1002/14651858.CD001689.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2021 Mar 29;3:CD001689. doi: 10.1002/14651858.CD001689.pub4. PMID: 24519568 Updated.
References
References to studies included in this review
Algahtani 2015 {published data only}
-
- Algahtani F, AL Dohami H, Abo-Harbesh S, Gader A, Aleem A. Thromboembolism prophylaxis after cesarean section (PRO-CS) trial. Thrombosis Research 2012;130(Suppl 1):S197.
-
- Algahtani F, Gader A, Aleem A, Al Dohami H, Garbesh S. Thromboembolism prophylaxis after cesarean section (pro-cs) trial. Haematologica 2013;98:749-50.
-
- Algahtani FH, Al-Dohami H, Abo-Harbesh S, Al-Gader A, Aleem A. Thromboembolism prophylaxis after cesarean section (PRO-CS) trial. Thrombosis Research 2015;135(Suppl 1):S71.
-
- NCT01321788. Venous Thromboembolism Prophylaxis Post Cesarean Section (PROCS). https://clinicaltrials.gov/ct2/show/NCT01321788 (first received 24 March 2011).
Burrows 2001 {published data only}
-
- Burrows RF, Gan ET, Gallus AS, Wallace EM, Burrows EA. A randomised, double blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thromboembolic events after caesarean section: a pilot study. British Journal of Obstetrics and Gynaecology 2001;108:835-9. - PubMed
Casele 2006 {published data only}
-
- Casele H, Haney E, James A, Rosene-Montella K, Carson M. Bone density changes in women receiving thromboprophylaxis in pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S14. - PubMed
-
- Casele H, Haney EI, James A, Rosene-Montella K, Carson M. Bone density changes in women who receive thromboprophylaxis in pregnancy. American Journal of Obstetrics and Gynecology 2006;195:1109-13. - PubMed
-
- Casele H. Thrombosis prophylaxis in pregnancy. www.enh.org (accessed 14 June 2005).
Cornette 2002 {published data only}
-
- Cornette J, Jacquemyn Y, Vercauteren M, Buytaert P. A randomised trial to compare the effect of pre- or postoperative nandroparin on blood loss during elective caesarean section. Phlebology 2002;17:67-9.
Cruz 2011 {published data only}
De Veciana 2001 {published data only}
-
- De Veciana M, Trail P, Dattel B, Slotnick RN, Abuhamad A. Dalteparin versus unfractionated heparin for prophylactic anticoagulation during pregnancy [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6):S182.
de Vries 2012 {published data only}ISRCTN87325378
-
- Abheiden C, Van Hoorn ME, Hague WM, Kostense PJ, Pampus MG, Vries J. Does low-molecular-weight heparin influence fetal growth or uterine and umbilical arterial doppler in women with a history of early-onset uteroplacental insufficiency and an inheritable thrombophilia? Secondary randomised controlled trial results. BJOG: an international journal of obstetrics and gynaecology 2016;123(5):797-805. - PubMed
-
- ISRCTN87325378. Low molecular weight heparin (Fragmin (R)) in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (FRUIT-study). http://www.isrctn.com/ISRCTN87325378 (first received 1 November 2005).
-
- NTR337. Low molecular weight heparin (Fragmin (R)) in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (FRUIT-study). Https://www.trialregister.nl/trial/299 (first received 2005).
-
- Vries J, Pampus MG, Hague WM, Bezemer PD, Joosten JH. Fractionated heparin in pregnant women with a history of uteroplacental insufficiency and thrombophilia, a randomized trial (The FRUIT Study). Reproductive Sciences 2011;18(3 Suppl 1):69A.
-
- Vries JI, Hague WM, Van Pampus MG, for the FRUIT-RCT Investigators. Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT: a reply to a rebuttal. Journal of Thrombosis & Haemostasis 2012;10(6):1196. - PubMed
Ellison 2001 {published data only}
-
- Ellison J, Thomson AJ, Conkie JA, McCall F, Walker ID, Greer IA. Thromboprophylaxis following caesarean section. A comparison of the antithrombotic properties of three low molecular weight heparins - dalteparin, enoxaparin and tinzaparin. Thrombosis and Haemostasis 2001;86:1374-8. - PubMed
-
- Ellison J, Thomson AJ, Walker ID, Greer IA. Comparisons of the anti-factor XA activities of three commonly used low molecular weight heparins following caesarean section. Scottish Medical Journal 2000;45(3):94-5.
-
- Ellison J, Thomson AJ, Walker ID, Greer IA. Thromboprophylaxis following caesarean section: comparison of the anti-factor Xa activities of dalteparin, enoxaparin and tinzaparin. British Journal of Obstetrics and Gynaecology 2000;107:823.
-
- Ellison J, Thomson AJ, Walker ID, Greer IA. Thromboprophylaxis following caesarean section: comparison of the anti-factor Xa activities of three commonly used low molecular weight heparins. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S39-S40.
Gates 2004a {published data only}
-
- Brocklehurst P, Thromboprophylaxis in Pregnancy Advisory Group. Thromboprophylaxis in pregnancy trials: apple, plum and peach. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):53.
-
- Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low molecular weight heparin. American Journal of Obstetrics and Gynecology 2004;191:1296-303. - PubMed
-
- Gates S, Brocklehurst P, Davis LJ. Antenatal thromboprophylaxis using low molecular weight heparin (enoxaparin) for women at risk of thromboembolic disease: multicentre placebo controlled randomised trial (APPLE) and systematic review. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S44.
-
- National Perinatal Epidemiology Unit. The APPLE Study. www.npeu.ox.ac.uk/trials/apple.html (first received 12 January 2001).
Gates 2004b {published data only}
-
- Brocklehurst P, Gates S. Randomised controlled trial (PEACH) and meta-analysis of thromboprophylaxis using low-molecular weight heparin (enoxaparin) after caesarean section. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S52.
-
- Brocklehurst P, Thromboprophylaxis in Pregnancy Advisory Group. Thromboprophylaxis in pregnancy trials: apple, plum and peach. British Journal of Obstetrics and Gynaecology 1998;105(Suppl 17):53.
-
- Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low molecular weight heparin. American Journal of Obstetrics and Gynecology 2004;191:1296-303. - PubMed
-
- National Perinatal Epidemiology Unit. The PEACH Study. www.npeu.ox.ac.uk/trials/peach.html (first received 12 January 2001).
Gibson 1998 {published data only}
-
- Gibson JL, Ekevall K, Walker I, Greer IA. Puerperal thromboprophylaxis: comparison of the anti-Xa activity of enoxaparin and unfractionated heparin. British Journal of Obstetrics and Gynaecology 1998;105:795-7. - PubMed
Hamersley 1998 {published data only}
-
- Hamersley S, Landy H. Low molecular weight heparin is associated with less peripartum blood loss than unfractionated heparin [abstract]. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S66.
Heilmann 1991 {published data only}
-
- Heilman L, Heitz R, Koch FU, Ose C. Perioperative thrombosis prophylaxis at the time of caesarean section: results of a randomised prospective comparative study with 6% hydroxyethyl starch 0.62 and low dose heparin [Die perioperative Thromboseprophylaxe beim Kaiserschnitt: Ergebnisse einer randomisierten prospektiven Vergleichsuntersuchung mit 6% Hydroxyathylstarke 0,62 und Low-dose-Heparin]. Zeitschrift fur Geburtshilfe und Perinatologie 1991;195:10-5. - PubMed
Heilmann 2007 {published data only}
-
- Heilmann L, Rath W, Pollow K, Bick RL. The rheological changes after cesarean section: the influence of low molecular weight or unfractionated heparin on the rheological properties of blood. Clinical Hemorheology and Microcirculation 2007;37:211-8. - PubMed
Heller 2016 {published data only}
-
- Heller J, Canner J, Wei Lum Y, Tsuchiya K. Compression stockings during pregnancy: essential or superfluous? A pilot study. Journal of Vascular Surgery: Venous and Lymphatic Disorders 2016;4(1):148.
-
- NCT01793194. Preventing the development of venous insufficiency in pregnant women through use of compression stockings: a randomized pilot study. http://www.clinicaltrials.gov/ct2/show/NCT01793194 (first received 21 May 2013).
Hill 1988 {published and unpublished data}
Howell 1983 {published data only}
-
- Howell R, Fidler J, Letsky E, Swiet M. The risks of antenatal subcutaneous heparin prophylaxis: a controlled trial. British Journal of Obstetrics and Gynaecology 1983;90:1124-8. - PubMed
Krauss 1994 {published data only}
-
- Krauss T, Rath W, Dittmer U, Kuhn W. Use of LMW heparin (Fragmin) for the prevention of thromboembolism in obstetrics [Thromboembolieprophylaxe mit niedermolekularen Heparin (Fragmin)in der Geburtshilfe]. Zeitschrift fur Geburtshilfe und Perinatologie 1994;198:120-5. - PubMed
O'Riordan 2008 {published data only}
-
- O'Riordan MN, Horgan R, Arya A, Quinn S, Gaolebale PA, Sarkar RK, et al. Pharmokinetics of low molecular weight heparins in the postpartum period. In: Irish Perinatal Society Annual Meetings; 2008 April 11-12; Cork, Ireland. 2008:10.
Pettila 1999 {published data only}
-
- Pettila V, Kaaja R, Leinonen P, Ekblad U, Kataja M, Ikkala E. Thromboprophylaxis with low molecular weight heparin (dalteparin) in pregnancy. Thrombosis Research 1999;96:275-82. - PubMed
-
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thrombosis and Haemostasis 2002;87(2):182-6. - PubMed
Reddick 2014 {published data only}
-
- Reddick KL, Smrtka MP, Grotegut CA, James AH, Brancazio LR, Swamy GK. The effects of intermittent pneumatic compression during cesarean delivery on fibrinolysis. American Journal of Perinatology 2014;31(9):735-40. - PubMed
Rodger 2014 {published data only}ISRCTN87441504
-
- Abou-Nassar K, Kovacs MJ, Kahn SR, Wells P, Doucette S, Ramsay T, et al. The effect of dalteparin on coagulation activation during pregnancy in women with thrombophilia. A randomized trial. Thrombosis and Haemostasis 2007;98(1):163-71. - PubMed
-
- Abou-Nassar K, Rodger M, Kovacs MJ, Doucette S, Tim R, Kahn S, et al. The effect of dalteparin on coagulation activation during pregnancy in women with thrombophilia: a randomised trial. Blood 2006;108(11 Pt 1):262. - PubMed
-
- NCT00967382. Thrombophilia in pregnancy prophylaxis study (TIPPS). http://www.clinicaltrials.gov/ct2/show/NCT00967382 (first received 30 January 2013).
-
- Rodger M, Hague WM, Kingdom J, Kahn SR, Karovitch A, Wells PS, et al. The Thrombophilia in Pregnancy Prophylaxis Study (TIPPS): a multi-national randomized trial of dalteparin vs. no dalteparin to prevent pregnancy complications in pregnant thrombophilic women. Journal of Thrombosis and Haemostasis 2013;11(Suppl 2):78.
-
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet 2014;384:1673-83. - PubMed
Rodger 2015 {published data only}
-
- NCT01274637. PROSPER: Postpartum prophylaxis for PE randomized control trial pilot. http://clinicaltrials.gov/show/NCT01274637 (first received 15 February 2011).
-
- Rodger MA, Phillips P, Kahn SR, James AH, Konkle BA. Low-molecular-weight heparin to prevent postpartum venous thromboembolism. A pilot randomised placebo-controlled trial. Thrombosis and Haemostasis 2015;113(1):212-6. - PubMed
Rodger 2016 {published data only}
-
- NCT01274637. PROSPER: Postpartum prophylaxis for PE randomized control trial pilot. http://clinicaltrials.gov/show/NCT01274637 (first received 15 February 2011).
-
- Rodger MA, Phillips P, Kahn SR, Bates S, McDonald S, Khurana R, et al. Low molecular weight heparin to prevent postpartum venous thromboembolism. A pilot study to assess the feasibility of a randomized open-label trial. Thrombosis Research 2016;142:17-20. - PubMed
Salim 2016 {published data only}
-
- Garmi G, Okopnik M, Zuarez-Easton S, Zafran N, Romano S, Salim R. Placental findings following adjusting enoxaparin dosage according to anti-fxa levels in thrombophilic women. A randomized controlled trial. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl 1):S457.
-
- Garmi G, Zafran N, Okopnik M, Gavish I, Romano S, Salim R. Placental pathological findings following adjusting enoxaparin dosage in thrombophilic women: secondary analysis of a randomized controlled trial. Thrombosis and Haemostasis 2019;119(1):87-91. - PubMed
-
- NCT01068795. Dose adjusting enoxaparin thromboprophylaxis dosage according to anti-factor Xa plasma levels improve pregnancy outcome. http://clinicaltrials.gov/show/NCT01068795 (first received 18 June 2013).
-
- Salim R, Nachum Z, Gavish I, Romano S, Braverman M, Garmi G. Adjusting enoxaparin dosage according to anti-FXa levels and pregnancy outcome in thrombophilic women. A randomised controlled trial. Thrombosis and Haemostasis 2016;116(4):687-95. - PubMed
Segal 1975 {published data only}
-
- Segal S, Sadovsky E, Weinstein D, Polishuk WZ. Prevention of postpartum venous thrombosis with low doses of heparin. European Journal of Obstetrics and Gynecology and Reproductive Biology 1975;5:273-6. - PubMed
Stephenson 2016 {published data only}
-
- NCT02070237. Comparing anti-XA levels in post-cesarean patients undergoing enoxaparin thromboprophylaxis. https://clinicaltrials.gov/ct2/show/NCT02070237 (first received 25 February 2014).
-
- Stephenson M, Serra A, Neeper J, Caballero D, McNulty J. Comparing anti-Xa levels in women with body mass index >/=35 post cesarean delivery undergoing enoxaparin thromboprophylaxis with weight-based dosing twice daily versus fixed dose 40 mg daily: a randomized, controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18.
-
- Stephenson ML, Serra AE, Neeper JM, Caballero DC, McNulty J. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. Journal of Perinatology 2016;36(2):95-9. - PubMed
van Hoorn 2016 {published data only}ISRCTN87325378
-
- Hoorn M, Hague WM, Pampus M, Bezemer D, de Vries J for the FRUIT investigators. Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: the FRUIT-RCT. European Journal of Obstetrics & Gynecology and Reproductive Biology 2016;197:168-73. - PubMed
Welti 1981 {published data only}
-
- Welti H. Prevention of thromboembolism by physiotherapy with and without low dose heparin in gynecology and obstetrics. Results of a controlled, randomized multicenter study [Prophylaxie thrombo-embolique par physiotherapie avec et sans heparine a faibles doses en gynecologie-obstetrique]. Revue Medicale de la Suisse Romande 1981;101(11):925-34. - PubMed
References to studies excluded from this review
Aina 2006 {published data only}
-
- Aina A, NCT00356434. Study of patient compliance and comfort using sequential compression devices and foot pumps for DVT prevention. https://clinicaltrials.gov/ct2/show/results/NCT00356434 (first received 26 July 2006).
Alalaf 2015 {published data only}
-
- Alalaf A, NCT01588171. Bemiparin versus enoxaparin as thromboprophylaxis following vaginal and abdominal deliveries: a prospective clinical trial. https://clinicaltrials.gov/ct2/show/NCT01588171 (first received 30 April 2012). - PMC - PubMed
Badawy 2008 {published data only}
-
- Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. Journal of Obstetrics and Gynaecology 2008;28(3):280-4. - PubMed
Blomback 1998 {published data only}
-
- Blomback M, Bremme K, Hellgren M, Lindberg H. A pharmacokinetic study of dalteparin (Fragmin) during late pregnancy. Blood Coagulation and Fibrinolysis 1998;9:343-50. - PubMed
Brenner 2005 {published data only}
-
- Brenner B, Bar J, Ellis M, Yarom I, Yohai D, Samueloff A, et al. Effects of enoxaparin on late pregnancy complications and neonatal outcome in women with recurrent pregnancy loss and thrombophilia: results from the live-enox study. Fertility and Sterility 2005;84(3):770-3. - PubMed
-
- Brenner B, Hoffman R, Carp H, Dulitsky M, Samueloff A, Yohai D, et al. Enoxaparin treatment improves the gestational outcome of pregnant women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study [abstract]. Blood 2003;102(11):16a.
-
- Brenner B, Hoffman R, Carp H, Dulitsky M, Samueloff A, Yohal D, et al. Efficacy and safety of two doses of enoxaparin in pregnant women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study [abstract]. Journal of Thrombosis and Haemostasis 2003;1(Suppl 1):OC084. - PubMed
-
- Brenner B, Hoffman R, Carp H, Dulitsky M, Younis J, LIVE-ENOX investigators. Efficacy and safety of two doses of enoxaparin in women with thrombophilia and recurrent pregnancy loss: the LIVE-ENOX study. Journal of Thrombosis and Haemostasis 2005;3:227-9. - PubMed
-
- Brenner B, for the LIVE-ENOX Investigators. Efficacy and safety of two doses of enoxaparin in pregnant women with thrombophilia and recurrent pregnancy loss. The LIVE-ENOX study [abstract]. Blood 2002;100(11 Pt 1):702a. - PubMed
de Jong 2015 {published data only}
Dendrinos 2007 {published data only}
-
- Dendrinos S, Kalogirou I, Makrakis E, Theodoridis T, Mahmound EA, Christopoulou-Cokkinou V, et al. Safety and effectiveness of tinzaparin sodium in the management of recurrent pregnancy loss. Clinical and Experimental Obstetrics and Gynecology 2007;34(3):143-5. - PubMed
Farquharson 2002 {published data only}
-
- Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstetrics and Gynecology 2002;100(3):408-13. - PubMed
Giancotti 2012 {published data only}
-
- Chistolini A, Torelli F, Giancotti A, Pignoloni P, Muto B, Cosimo C, et al. Recurrent fetal loss: prospective evaluation of the efficacy of three different thromboprophylaxis regimens: aspirin versus low molecular weight heparin versus low molecular weight heparin plus aspirin [abstract]. Hematology Journal 2006;91(Suppl 1):146.
-
- Giancotti A, Torre RL, Spagnuolo A, D'Ambrosio V, Cerekja A, Piazze J, et al. Efficacy of three different antithrombotic regimens on pregnancy outcome in pregnant women affected by recurrent pregnancy loss. Journal of Maternal-Fetal and Neonatal Medicine 2012;25(7):1191-4. - PubMed
Gris 2010 {published data only}
-
- Gris JC, Chauleur C, Faillie JL, Baer G, Mares P, Fabbro-Peray P, et al. Enoxaparin for the secondary prevention of placental vascular complications in women with abruptio placentae: the pilot randomised controlled NOH-AP trial. Thrombosis and Haemostasis 2010;104(4):771-9. - PubMed
Gris 2011 {published data only}
-
- Gris JC, Chauleur C, Mares P, Nouvellon E, Mercier E, Bouvier S, et al. Enoxaparin for the secondary prevention of placental complications in women with previous severe pre-eclampsia: the pilot randomised controlled NOH-PE study. Journal of Thrombosis and Haemostasis : JTH 2011;9(Suppl 2):753.
-
- Gris JC, Chauleur C, Molinari N, Mares P, Fabbro-Peray P, Quere I, et al. Addition of enoxaparin to aspirin for the secondary prevention of placental vascular complications in women with severe pre-eclampsia: the pilot randomised controlled NOH-PE trial. Thrombosis and Haemostasis 2011;106(6):1053-61. - PubMed
Guven 2014 {published data only}
-
- Guven D, Bakay K, Kocak I, Ozdemir A. Effectiveness of bemiparin sodium for preventing pregnancy loss in patients with MTHFR C677T mutation and habitual abortus. Open Journal of Obstetrics and Gynecology 2014;4:930-4.
Harenberg 1993 {published data only}
-
- Harenberg J, Schneider D, Heilmann L, Wolf H. Lack of anti-factor Xa activity in umbilical cord vein samples after subcutaneous administration of heparin or low molecular mass heparin in pregnant women. Haemostasis 1993;23:314-20. - PubMed
Kaandorp 2010 {published data only}
-
- Kaandorp SP, Goddijn M, Post JA, Hutten BA, Verhoeve HR, Hamulyák K, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. New England Journal of Medicine 2010;362(17):1586-96. - PubMed
-
- Middeldorp S. Aspirin and/or low molecular weight heparin for women with unexplained recurrent miscarriage and/or intra-uterine fetal death. Netherlands Trial Register (http://www.trialregister.nl) (first received 1 November 2005).
Kamin 2008 {published data only}
-
- Kamin G, Rogenhofer N, Pildner v Steinberg S, Neuhoffer A, Seeger S, et al. Therapy with dalteparin for habitual abortion - presentation of the ETHiG II-Studie [Therapie mit Dalteparin bei habitueller Abortneigung - Vorstellung der ETHiG II-Studie]. Geburtshilfe und Frauenheilkunde 2008;68:S51.
-
- Rogenhofer N, Markoff A, Wagner A, Klein H-G, Petroff D, Schleussner E, et al. Lessons from the EThIGII trial: Proper putative benefit assessment of low-molecular-weight heparin treatment in M2/ANXA5 haplotype carriers. Clinical and Applied Thrombosis/Hemostasis 2017;23(1):27-33. - PubMed
-
- Schleussner E, Bohlmann M, Kamin G, Rogenhofer N, Seeger S, Toth B. Low molecular weight heparin for the prevention of habitual abortion - introduction of the multicentre study EThIG2 and discussion of the current data [Niedermolekulares heparin zur pravention habitueller aborte - vorstellung der multizentrischen EThIG2-studie und diskussion der aktuellen datenlage]. Archives of Gynecology and Obstetrics 2012;286(Suppl 1):S220.
-
- Schleussner E, Kamin G, Seeliger G, Rogenhofer N, Toth B. Low-molecular-weight heparin in recurrent pregnancy loss-Results of the ETHIG II study. Thrombosis Research 2013;131(Suppl 1):S73.
Kutteh 1996a {published data only}
-
- Kutteh WH, Ermel LD. A clinical trial for the treatment of antiphospholipid antibody-associated recurrent pregnancy loss with lower dose heparin and aspirin. American Journal of Reproductive Immunology 1996;35:402-7. - PubMed
Kutteh 1996b {published data only}
-
- Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. American Journal of Obstetrics and Gynecology 1996;174(5):1584-9. - PubMed
Langer 2013 {published data only}
-
- Langer E, Fiedler A, Schleusner E, Schlembach D. Placental volume in women under treatment with recurrent miscarriages. Ultraschall in der Medizin, Supplement 2013;34(Suppl 1):WS_SL6_05.
Laskin 2007 {published data only}
-
- Laskin CA, NCT00564174. A randomized controlled trial comparing low molecular weight heparin and aspirin to aspirin alone in women with unexplained recurrent pregnancy loss. https://clinicaltrials.gov/ct2/show/NCT00564174 (first received 27 November 2007).
-
- Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. Journal of Rheumatology 2009;36(2):279-87. - PubMed
Milic 2018 {published data only}
-
- Milic D, Zivic S, Bogdanovic D, Jovanovic M. Compression stockings in the prevention of venous disorders in pregnancy. Phlebology 2018;33(10):709.
Noble 2005 {published data only}
-
- Noble LS, Kutteh WS, Lashey N, Franklin RD, Herrada J. Antiphospholipid antibodies associated with recurrent pregnancy loss: prospective, multicenter, controlled pilot study comparing treatment with low-molecular-weight heparin versus unfractionated heparin. Fertility and Sterility 2005;83(3):684-90. - PubMed
Pyregov 2012 {published data only}
-
- Pyregov A, Shifman E, Shestakova O, Puchko T, Baranov I. Influence of enoxaparine on serum endotoxin concentration in puerpera after abdominal delivery. British Journal of Anaesthesia 2012;108:ii212-3.
Rai 1997 {published data only}
-
- Cohen H. Randomized trial of aspirin versus aspirin and heparin in pregnant women with the antiphospholipid syndrome. Annales de Medicine Interne 1996;147 Suppl 1:44. - PubMed
Ratiu 2009 {published data only}
-
- Ratiu A, Motoc A, Pascut D, Crisan DC, Anca T, Pascut M. Compression and walking compared with bed rest in the treatment of proximal deep venous thrombosis during pregnancy. Revista Medico-Chirurgicala a Societatii de Medici Si Naturalisti Din Iasi 2009;113(3):795-8. - PubMed
Rey 2009 {published data only}
-
- Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. Journal of Thrombosis and Haemostasis 2009;7(1):58-64. - PubMed
-
- Rey E. Dalteparin in prevention of recurrence of severe obstetrical complications in women without thrombophilia. Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S46.
Rodger 2017 {published data only}
-
- Rodger M, NCT03100123. A pilot study assessing the feasibility of a randomized controlled trial evaluating aspirin versus low-molecular-weight heparin (lmwh) and aspirin in women with antiphospholipid syndrome and pregnancy loss. https://clinicaltrials.gov/ct2/show/NCT03100123 (first received 4 April 2017).
Samantha 2013 {published data only}
-
- Samantha P, Barillari G, Venturelli U, Turello M. Thromboprophylaxis with two different dosages of lmwh (calcic nadroparin) in pregnant women with thrombophilia: a single center experience. Journal of Thrombosis and Haemostasis 2013;11:96-7.
Schleussner 2015 {published data only}
-
- Schleussner E, Kamin G, Seliger G, Rogenhofer N, Ebner S, Toth B, et al. Low-molecular-weight heparin for women with unexplained recurrent pregnancy loss: a multicenter trial with a minimization randomization scheme. Annals of Internal Medicine 2015;162(9):601-9. - PubMed
Stephenson 2004 {published data only}
-
- Stephenson MD, Ballem PJ, Tsang P, Purkiss S, Ensworth S, Houlihan E, et al. Treatment of antiphospholipid antibody syndrome (aps) in pregnancy: a randomized pilot trial comparing low molecular weight heparin to unfractionated heparin. Journal of Obstetrics and Gynaecology Canada 2004;26(8):729-34. - PubMed
Thaler 2004 {published data only}
-
- Thaler I, Brenner B. Efficacy of enoxaparin for improving pregnancy outcomes and uteroplacental blood flow in women with thrombophilia and recurrent pregnancy loss [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S7.
Tulppala 1997 {published data only}
-
- Tulppala M, Marttunen M, Soderstrom-Anttila V, Foudila T, Ailus K, Palosuo T, et al. Low-dose aspirin in prevention of miscarriage in women with unexplained or autoimmune related recurrent miscarriage: effect of prostacyclin and thromboxane A2 production. Human Reproduction 1997;12(7):1567-72. - PubMed
Visser 2011 {published data only}
-
- Visser J, Ulander V, Bloemenkamp K, Kaaja R. A randomised controlled multicenter study: the effect of enoxaparin and/or aspirin on prevention of recurrent miscarriage in women with or without thrombophilia, HABENOX-study. International Journal of Gynecology and Obstetrics 2009;107(Suppl 2):S371.
-
- Visser J, Ulander VM, Helmerhorst FM, Lampinen K, Morin-Papunen L, Bloemenkamp KW, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia - HABENOX*: a randomised multicentre trial. Thrombosis and Haemostasis 2011;105(2):295-301. - PubMed
References to studies awaiting assessment
Abdolvand 2019 {published data only}
-
- Abdolvand M, Aleyasin A, Javadi MR, Solduzian M, Hosseini SH, Ziaei Z, et al. Comparison of efficacy and safety of two different enoxaparin products in prevention of venous thromboembolism following major obstetric-gynecological surgeries: an open-label randomized clinical trial. Iranian Journal of Pharmaceutical Research 2019;18(4):2172-9. [CENTRAL: CN-02074323] [EMBASE: 2003440151] - PMC - PubMed
Dittmer 1991 {published data only}
-
- Dittmer U, Rath W, Schrader J, Zuchner C, Scheler F, Kuhn W. Prevention of deep vein thrombosis (DVT) in pregnancy, after caesarean section and after gynaecological abdominal surgery. A comparison between low molecular weight heparin (LMW) and standard heparin (UFH) [abstract]. Annals of Haematology 1991;62 Suppl 1:A40.
Ganer 2020 {published data only}
-
- Ganer Herman H, Kleiner I, Tairy D, Gonen N, Ben Zvi M, Kovo M, et al. Effect of digital step counter feedback on mobility after cesarean delivery: a randomized controlled trial. Obstetrics and Gynecology 2020;135(6):1345-52. [CENTRAL: CN-02131345] [EMBASE: 631910090] [PMID: ] - PubMed
-
- Herman HG, Kleiner I, Tairy D, Gonen N, Ben ZM, Kovo M, et al. 623: improving post-Cesarean mobility with personalized feedback using digital step counters - a randomized controlled trial. American Journal of Obstetrics and Gynecology 2020;222(1):S397-8. [CENTRAL: CN-02075395] [EMBASE: 2004455439]
Movahedi 2020 {published data only}
-
- Movahedi M, Motamedi M, Sajjadieh A, Bahrami P, Saeedi M. Pregnancy outcome in women with mechanical prosthetic heart valves at their first trimester of pregnancy treated with unfractionated heparin (UFH) or enoxaparin: a randomized clinical trial. Journal of Cardiovascular and Thoracic Research 2020;12(3):209-13. [CENTRAL: CN-02194966] [EMBASE: 632980316] [PMID: ] - PMC - PubMed
Nagornaya 2012 {published data only}
-
- Nagornaya V, Gonta R, Nickolay K, Pokhilchenko M. The risk of venous thromboembolism and its prevention in obstetrics. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S796.
NCT02856295 {published data only}
-
- NCT02856295. anti10a levels in women treated with LMWH in the postpartum period. Https://clinicaltrials.gov/show/NCT02856295 (first received 2016 August 04). [CENTRAL: CN-01592274]
NCT04305756 {published data only}
-
- NCT04305756. Impact of prophylactic low-molecular weight heparin dosing on clotting parameters following cesarean delivery. Https://clinicaltrials.gov/show/NCT04305756 (first received 2020 March 12). [CENTRAL: CN-02089003]
NCT04635839 {published data only}
-
- NCT04635839. Heparin Prophylaxis Dosing for antepartum hospitalizations (HEPDOSE). Https://clinicaltrials.gov/show/NCT04635839 (first received 2020 Nov 19). [CENTRAL: CN-02205733]
References to ongoing studies
Dargaud 2018 {published data only}
-
- Dargaud Y, NCT03659708. Prospective multicentre randomized clinical trial on the management of pregnancies with high risk of venous thrombosis. https://clinicaltrials.gov/ct2/show/NCT03659708 (first received 6 September 2018).
Heller 2016b {published data only}
-
- Heller J. Compression stocking use in multiparous women during pregnancy & its effects on chronic venous insufficiency. Phlebology 2016;31(2 Suppl):42-3.
NCT00225108 {published data only}
-
- NCT00225108. The STOP CLOT pilot study: study of low molecular weight heparin in high risk postpartum women following cesarean section (ongoing trial). http://clinicaltrials.gov/show/NCT00225108 (first received 21 March 2006).
NCT00878826 {published data only}
-
- NCT00878826. Prophylactic enoxaparin dosing for prevention of venous thromboembolism in pregnancy. http://clinicaltrials.gov/ct2/show/record/NCT00878826 (first received 31 March 2013).
NCT01019655 {published data only}
-
- NCT01019655. Heparin for pregnant with thrombophilia. http://clinicaltrials.gov/show/NCT01019655 (first received 29 July 2012).
NCT01828697 {published data only}
-
- Bistervels I, Bleker S, Middeldorp S, Buchmuller A, Decousus H, Chauleur C, et al. Induced delivery and neuraxial anesthesia in pregnant women using thromboprophylaxis: data from the highlow study. Research and Practice in Thrombosis and Haemostasis 2017;1:41-2.
-
- Bistervels I, Buchmüller A, Bleker S, Chauleur C, Ni Ainle F, Donnelly J, et al. Neuraxial anesthesia in pregnant women using thrombosis prophylaxis: data from the Highlow study. Thrombosis Research 2017;Conference: 7th International Symposium on Women's Health Issues in Thrombosis and Haemostasis. Spain. 151(Suppl 1):S131-2.
-
- Bistervels I, Buchmuller A, Ni Ainle F, Bleker S, Chauleur C, Donnelly J, et al. Management of anticoagulant therapy around delivery: results from the ongoing highlow study. Research and Practice in Thrombosis and Haemostasis 2019;3:866-7. [CENTRAL: CN-01978640] [EMBASE: 628813887]
-
- Bleker SM, Buchmuller A, Chauleur C, Ainle FN, Donnelly J, Verhamme P, et al. Low-molecular-weight heparin to prevent recurrent venous thromboembolism in pregnancy: Rationale and design of the Highlow study, a randomised trial of two doses. Thrombosis Research 2016;144:62-8. - PubMed
-
- Bleker SM, Ingrid BM, Buchmuller A, Chauleur C, Ainle FN, Donnelly J, et al. Interim report of the highlow study: a randomized controlled trial comparing two doses of low molecular weight heparin for the prevention of pregnancy-associated recurrent venous thromboembolism. Blood 2016;128(22):1444.
NCT04153760 {published data only}
-
- NCT04153760. Pilot PARTUM Trial: Postpartum Aspirin to reduce thromboembolism undue morbidity (PARTUM). https://clinicaltrials.gov/ct2/show/NCT04153760 (first received 19 August 2020).
Additional references
Abbasi 2014
-
- Abbasi N, Balayla J, Laporta DP, Kezouh A, Abenhaim HA. Trends, risk factors and mortality among women with venous thromboembolism during labour and delivery: a population based study of 8 million births. Archives of Gynecology and Obstetrics 2014;289(2):275-84. - PubMed
ACOG 2018
-
- American College of Obstetricians and Gynecologists. Thromboembolism in pregnancy. ACOG Practice Bulletin No. 196. Obstetrics and Gynecology 2018;132:e1-17. - PubMed
Andrew 2020
-
- Andrew L, Ni Ainle F, Blondon M, Rodger M, Skeith L. Preventing postpartum venous thromboembolism: a call to action to reduce undue maternal morbidity and mortality. Thrombosis Research 2020;193:190-7. - PubMed
Ansell 2004
-
- Ansell J, Bergqvist D. Current options in the prevention of thromboembolic disease. Drugs 2004;64(Suppl 1):1-5. - PubMed
Antony 2017
-
- Antony KM, Racusin DA, Aagaard K, Dildy GA. III Maternal physiology. In: Gabbe SG, Niebyl JR, Simpson JO, Landon MB, Galan HL, Jauniaux ER et al, editors(s). Obstetrics: Normal and Problem Pregnancies. 7th edition. Philadelphia: Elsevier, 2017.
Bates 2012
-
- Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy,and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed:American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012;141(2 Suppl):e691S–e6736. - PMC - PubMed
Bauersachs 2009
-
- Bauersachs RM. Treatment of venous thromboembolism during pregnancy. Thrombosis Research 2009;123(Suppl 2):S45-S50. - PubMed
Blanco‐Molina 2010
-
- Blanco-Molina A, Rota L, Di Micco P, Brenner B, Tujilo-Santos J, Ruiz-Gamietea A, et al. Venous thromboembolism during pregnancy, postpartum or during contraceptive use. RIETE Investigators. Thombosis and Haemostasis 2010;103:306-11. - PubMed
Brenner 2003
-
- Brenner B. Antithrombotic prophylaxis for women with thrombophilia and pregnancy complications--Yes. Journal of Thrombosis and Haemostasis 2003;1(10):2070-2. - PubMed
D'Souza 2020
-
- D'Souza R, Malhamé I, Teshler L, Acharya G, Hunt BJ, McLintock C. A critical review of the pathophysiology of thrombotic complications and clinical practice recommendations for thromboprophylaxis in pregnant patients with COVID-19. Acta Obstetricia et Gynecologica Scandinavica 2020;Jul 17 [Epub ahead of print]. [DOI: 10.1111/aogs.13962] - DOI - PMC - PubMed
de Jong 2014
Di Nisio 2017
-
- Di Nisio M, Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet 2017;388(10063):3060-73. - PubMed
Dodd 2013
Ellis‐Kahana 2020
Friedman 2016
-
- Friedman A, Ananth C. Obstetrical venous thromboembolism: Epidemiology and strategies for prophylaxis. Seminars in Perinatology 2016;40:81-6. - PubMed
Goldhaber 2012
-
- Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835-46. - PubMed
Greer 2012
-
- Greer IA. Thrombosis in pregnancy: updates in diagnosis and management. Hematologic Diseases in Pregnancy 2012;2012:203-5. - PubMed
Hall 1980
-
- Hall JG, Pauli RM, Wilson KM. Maternal and fetal sequelae of anticoagulation during pregnancy. American Journal of Medicine 1980;68:122-40. - PubMed
Hamulyák 2020
-
- Hamulyák EN, Scheres LJ, Marijnen MC, Goddijn M, Middeldorp S. Aspirin or heparin or both for improving pregnancy outcomes in women with persistent antiphospholipid antibodies and recurrent pregnancy loss. Cochrane Database of Systematic Reviews 2020, Issue 5. Art. No: CD012852. [DOI: 10.1002/14651858.CD012852] - DOI - PMC - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Jacobsen 2008
-
- Jacobsen A, Skjeldestad FE, Sandset PM. Incidence and risk patterns of venous thromboembolism in pregnancy and puerperium - a register-based case-control study. American Journal of Obstetrics andGynecology 2008;198(233):e1-7. - PubMed
James 2006
-
- James AH, Jamison MG, Brancazio LR. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. American Journal of Obstetrics and Gynecology 2006;194:1311–5. - PubMed
Knight 2008
-
- Knight M. Antenatal pulmonary emboism: risk factors, management and outcomes. BJOG: an international journal of obstetrics and gynaecology 2008;115:453-61. - PubMed
Letsky 1997
-
- Letsky EA. Peripartum prophylaxis of thromboembolism. Bailliere's Clinical Obstetrics and Gynaecology 1997;11:523-43. - PubMed
Middeldorp 2003
-
- Middeldorp S. Antithrombotic prophylaxis for women with thrombophilia and pregnancy complications--No. Journal of Thrombosis and Haemostasis 2003;1(10):2073-4. - PubMed
Nelson‐Piercy 1997
-
- Nelson-Piercy C. Hazards of heparin: allergy, heparin-induced thrombocytopenia and osteoporosis. Bailliere's Clinical Obstetrics and Gynaecology 1997;11:489-509. - PubMed
NHMRC 2009
-
- National Health and Medical Research Council. Clinical Practice Guideline for the Prevention of Venous Thromboembolism (Deep Vein Thrombosis and Pulmonary Embolism) in Patients Admitted to Australian Hospitals. Melbourne: National Health and Medical Research Council, 2009. - PubMed
NICE 2018
-
- NICE 2018. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism (NG89). www.nice.org.uk/guidance/ng89 2018. - PubMed
Okoroh 2012
Orme 1977
Palmerola 2015
-
- Palmerola KL, D' Alton ME, Brock CO, Friedman AM. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG: an international journal of obstetrics and gynaecology 2016;123(13):2157-62. - PubMed
Paull 1987
-
- Paull J. A prospective study of dextran-induced anaphylactoid reactions in 5745 patients. Anaesthesia and Intensive Care 1987;15:163-7. - PubMed
PEP Trial 2000
-
- PEP Trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin. Lancet 2000;355:1295-302. - PubMed
RCOG 2015
-
- The Royal College of Obstetricians and Gynaecologists. Reducing the risk of thrombosis and embolism during pregnancy and puerperium. Green-top guideline. https://www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg37a/ 2015:1-40.
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014.
Roberge 2017
-
- Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. American Journal of Obstetrics & Gynecology 2017;February:110-120. - PubMed
Rolnik 2017
-
- Rolnik DL, Wright D, Poon LC, O' Gorman N, Syngelak A, Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. New England Journal of Medicine 2017;177(7):613-22. - PubMed
Simpson 2001
-
- Simpson EL, Lawrenson RA, Nightingale AL, Farmer RD. Venous thromboembolism in pregnancy and the puerperium: incidence and additional risk factors from a London perinatal database. British Journal of Obstetrics and Gynaecology 2001;108(1):56-60. - PubMed
Walker 2003
Wu 2005
-
- Wu O, Robertson L, Twaddle S, Lowe G, Clark P, Walker I, et al. Screening for thrombophilia in high-risk situations: a meta-analysis and cost-effectiveness analysis. British Journal of Haematology 2005;131(1):80-90. - PubMed
References to other published versions of this review
Bain 2014
Gates 2002a
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

