Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer -Association With Patient and Disease Characteristics and Effect on Prognosis
- PMID: 33780288
- PMCID: PMC8274805
- DOI: 10.1200/JCO.20.01200
Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer -Association With Patient and Disease Characteristics and Effect on Prognosis
Abstract
Purpose: Among patients with metastatic breast cancer (mBC), the frequency of germline mutations in cancer susceptibility genes and the clinical relevance of these mutations are unclear. In this study, a prospective cohort of patients with mBC was used to determine mutation rates for breast cancer (BC) predisposition genes, to evaluate the clinical characteristics of patients with mutations, and to assess the influence of mutations on patient outcome.
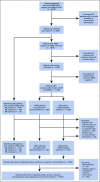
Patients and methods: Germline DNA from 2,595 patients with mBC enrolled in the prospective PRAEGNANT registry was evaluated for mutations in cancer predisposition genes. The frequencies of mutations in known BC predisposition genes were compared with results from a prospective registry of patients with nonmetastatic BC sequenced using the same QIAseq method and with public reference controls. Associations between mutation status and tumor characteristics, progression-free survival, and overall survival were assessed.
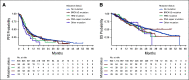
Results: Germline mutations in 12 established BC predisposition genes (including BRCA1 and BRCA2) were detected in 271 (10.4%) patients. A mutation in BRCA1 or BRCA2 was seen in 129 patients (5.0%). BRCA1 mutation carriers had a higher proportion of brain metastasis (27.1%) compared with nonmutation carriers (12.8%). Mutations were significantly enriched in PRAEGNANT patients with mBC compared with patients with nonmetastatic BC (10.4% v 6.6%, P < .01). Mutations did not significantly modify progression-free survival or overall survival for patients with mBC.
Conclusion: Multigene panel testing may be considered in all patients with mBC because of the high frequency of germline mutations in BRCA1/2 and other BC predisposition genes. Although the prognosis of mutation carriers and nonmutation carriers with mBC was similar, differences observed in tumor characteristics have implications for treatment and for future studies of targeted therapies.
Conflict of interest statement
Figures
References
-
- Condorelli R, Mosele F, Verret B, et al. Genomic alterations in breast cancer: Level of evidence for actionability according to ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT) Ann Oncol 30365–3732019 - PubMed
-
- Robson M, Im SA, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation N Engl J Med 377523–5332017 - PubMed
-
- Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy J Clin Oncol 28375–3792010 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

