Sensitive electrochemical biosensor combined with isothermal amplification for point-of-care COVID-19 tests
- PMID: 33780853
- PMCID: PMC7970423
- DOI: 10.1016/j.bios.2021.113168
Sensitive electrochemical biosensor combined with isothermal amplification for point-of-care COVID-19 tests
Abstract
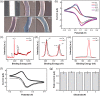
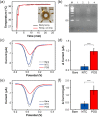
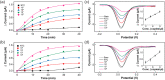
We report an electrochemical biosensor combined with recombinase polymerase amplification (RPA) for rapid and sensitive detection of severe acute respiratory syndrome coronavirus 2. The electrochemical biosensor based on a multi-microelectrode array allows the detection of multiple target genes by differential pulse voltammetry. The RPA reaction involves hybridization of the RPA amplicon with thiol-modified primers immobilized on the working electrodes, which leads to a reduction of current density as amplicons accumulate. The assay results in shorter "sample-to-answer" times than conventional PCR without expensive thermo-cycling equipment. The limits of detection are about 0.972 fg/μL (RdRP gene) and 3.925 fg/μL (N gene), which are slightly lower than or comparable to that of RPA assay results obtained by gel electrophoresis without post-amplification purification. The combination of electrochemical biosensors and the RPA assay is a rapid, sensitive, and convenient platform that can be potentially used as a point-of-care test for the diagnosis of COVID-19.
Keywords: COVID-19; Coronavirus; Electrochemical detection method; Recombinase polymerase amplification; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Rapid electrochemical detection of coronavirus SARS-CoV-2.Nat Commun. 2021 Feb 5;12(1):802. doi: 10.1038/s41467-021-21121-7. Nat Commun. 2021. PMID: 33547323 Free PMC article.
-
Isothermal recombinase polymerase amplification-lateral flow detection of SARS-CoV-2, the etiological agent of COVID-19.J Virol Methods. 2021 Oct;296:114227. doi: 10.1016/j.jviromet.2021.114227. Epub 2021 Jul 2. J Virol Methods. 2021. PMID: 34224752 Free PMC article.
-
Specific lateral flow detection of isothermal nucleic acid amplicons for accurate point-of-care testing.Biosens Bioelectron. 2023 Feb 15;222:114989. doi: 10.1016/j.bios.2022.114989. Epub 2022 Dec 15. Biosens Bioelectron. 2023. PMID: 36538868
-
Application of recombinase polymerase amplification with lateral flow assay to pathogen point-of-care diagnosis.Front Cell Infect Microbiol. 2024 Nov 18;14:1475922. doi: 10.3389/fcimb.2024.1475922. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39624267 Free PMC article. Review.
-
Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example.Biosens Bioelectron. 2022 Jun 15;206:114109. doi: 10.1016/j.bios.2022.114109. Epub 2022 Feb 26. Biosens Bioelectron. 2022. PMID: 35245867 Review.
Cited by
-
Next-Generation Microfluidics for Biomedical Research and Healthcare Applications.Biomed Eng Comput Biol. 2023 Nov 27;14:11795972231214387. doi: 10.1177/11795972231214387. eCollection 2023. Biomed Eng Comput Biol. 2023. PMID: 38033395 Free PMC article. Review.
-
Electroanalytical Paper-Based Nucleic Acid Amplification Biosensors with Integrated Thread Electrodes.Anal Chem. 2021 Oct 26;93(42):14187-14195. doi: 10.1021/acs.analchem.1c02900. Epub 2021 Oct 14. Anal Chem. 2021. PMID: 34648274 Free PMC article.
-
MXene-Based Electrochemical Biosensors: Advancing Detection Strategies for Biosensing (2020-2024).Biosensors (Basel). 2025 Feb 20;15(3):127. doi: 10.3390/bios15030127. Biosensors (Basel). 2025. PMID: 40136924 Free PMC article. Review.
-
The role of electrochemical biosensors in SARS-CoV-2 detection: a bibliometrics-based analysis and review.RSC Adv. 2022 Aug 12;12(35):22592-22607. doi: 10.1039/d2ra04162f. eCollection 2022 Aug 10. RSC Adv. 2022. PMID: 36105989 Free PMC article. Review.
-
Nanomaterials for IoT Sensing Platforms and Point-of-Care Applications in South Korea.Sensors (Basel). 2022 Jan 13;22(2):610. doi: 10.3390/s22020610. Sensors (Basel). 2022. PMID: 35062576 Free PMC article. Review.
References
-
- Akanda M.R., Sohail M., Aziz M.A., Kawde A.N. Electroanalysis. 2016;28:408–424.
-
- Akhavan O., Ghaderi E., Rahighi R. ACS Nano. 2012;6:2904–2916. - PubMed
-
- Akhavan O., Ghaderi E., Rahighi R., Abdolahad M. Carbon N. Y. 2014;79:654–663.
-
- Aravamudhan S., Kumar A., Mohapatra S., Bhansali S. Biosens. Bioelectron. 2007;22:2289–2294. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

