Clinical predictors of cardiac toxicity in HER2-positive early breast cancer patients treated with adjuvant s.c. versus i.v. trastuzumab
- PMID: 33780903
- PMCID: PMC8022886
- DOI: 10.1016/j.breast.2021.03.004
Clinical predictors of cardiac toxicity in HER2-positive early breast cancer patients treated with adjuvant s.c. versus i.v. trastuzumab
Abstract
Background: Few data are available about real-life cardiotoxicity associated with s.c. versus i.v. trastuzumab treatment of early-stage, HER2-positive breast cancer, and little is known about its predisposing factors.
Patients and methods: We retrospectively reviewed data of 363 adult patients treated with adjuvant trastuzumab for HER2-positive breast cancer. Univariate statistical analysis was performed, and a multivariable logistic model was developed to identify independent risk factors of cardiac toxicity.
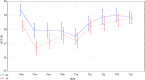
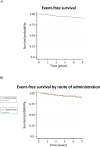
Results: Within 5 years, the overall incidence of events meeting our criteria was 11.8%, and an early discontinuation of trastuzumab was recorded in 20 patients (5.5%). No cases of congestive heart failure occurred, neither multiple events per patient were observed. A total of 184 patients received i.v. and 179 received s.c. trastuzumab. Compared with the s.c. formulation, a higher cardiotoxicity rate for the i.v. administration (15.2% vs 8.4%) was found, and particularly in those patients with cardiovascular risk factors (19.3% vs 8.7%), at the univariate and multivariate analyses. Although more patients with prior anthracycline-based chemotherapy experienced cardiac events, the association of this therapy with cardiac events was not significant. The incidence of cardiac events was not influenced by anthropometric data (e.g. body mass index) or a diagnosis of diabetes mellitus. 5-year event-free survival was 91.7% in the overall population; event-free survival rates were similar between the s.c. and the i.v. groups.
Conclusion: Our study shows a more favorable safety profile of s.c. versus i.v trastuzumab administration. The use of s.c. trastuzumab could be advisable in at-risk patients.
Keywords: Cardiac toxicity; Early breast cancer; HER2-positive; Intravenous; Subcutaneous; Trastuzumab.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Armando Santoro has received honoraria from BMS, AstraZeneca, MSD, Lilly, Bayer, Takeda, Roche, Mundipharma, Novartis, Servier, Amgen, ArQule, Celgene, Incyte, AbbVie, Gilead, Pfizer, Daiichi, Sandoz, and Sanofi. Rita De Sanctis has received honoraria form EISAI, Kyowa Kirin and Novartis. The other authors have no funding and conflicts of interests to disclose.
Figures
References
-
- Piccart-Gebhart M.J., Procter M., Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
-
- Cameron D., Piccart-Gebhart M.J., Gelber R.D., Procter M. Herceptin Adjuvant (HERA) Trial Study Team. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205. - PMC - PubMed
-
- Niraula S., Gyawali B. Optimal duration of adjuvant trastuzumab in treatment of early breast cancer: a meta-analysis of randomized controlled trials. Breast Canc Res Treat. 2019;173:103–109. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous