Isotope ratio-based quantification of carbon assimilation highlights the role of plastidial isoprenoid precursor availability in photosynthesis
- PMID: 33781281
- PMCID: PMC8008545
- DOI: 10.1186/s13007-021-00731-8
Isotope ratio-based quantification of carbon assimilation highlights the role of plastidial isoprenoid precursor availability in photosynthesis
Abstract
Background: We report a method to estimate carbon assimilation based on isotope ratio-mass spectrometry (IRMS) of 13CO2 labeled plant tissue. Photosynthetic carbon assimilation is the principal experimental observable which integrates important aspects of primary plant metabolism. It is traditionally measured through gas exchange. Despite its centrality in plant research, gas exchange performs poorly with rosette growth habits typical of Arabidopsis thaliana, mutant lines with limited biomass, and accounts poorly for leaf shading.
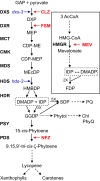
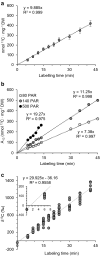
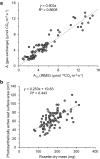
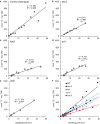
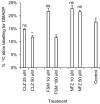
Results: IRMS-based carbon assimilation values from plants labeled at different light intensities were compared to those obtained by gas exchange, and the two methods yielded similar values. Using this method, we observed a strong correlation between 13C content and labeling time (R2 = 0.999) for 158 wild-type plants labeled for 6 to 42 min. Plants cultivated under different light regimes showed a linear response with respect to carbon assimilation, varying from 7.38 nmol 13C mg-1 leaf tissue min-1 at 80 PAR to 19.27 nmol 13C mg-1 leaf tissue min-1 at 500 PAR. We applied this method to examine the link between inhibition of the 2C-methyl-D-erythritol-4-phosphate (MEP) pathway and suppression of photosynthesis. A significant decrease in carbon assimilation was observed when metabolic activity in the MEP pathway was compromised by mutation or herbicides targeting the MEP pathway. Mutants affected in MEP pathway genes 1-DEOXY-D-XYLULOSE 5-PHOSPHATE SYNTHASE (DXS) or 1-HYDROXY-2-METHYL-2-(E)-BUTENYL 4-DIPHOSPHATE SYNTHASE (HDS) showed assimilation rates 36% and 61% lower than wild type. Similarly, wild type plants treated with the MEP pathway inhibitors clomazone or fosmidomycin showed reductions of 52% and 43%, respectively, while inhibition of the analogous mevalonic acid pathway, which supplies the same isoprenoid intermediates in the cytosol, did not, suggesting inhibition of photosynthesis was specific to disruption of the MEP pathway.
Conclusions: This method provides an alternative to gas exchange that offers several advantages: resilience to differences in leaf overlap, measurements based on tissue mass rather than leaf surface area, and compatibility with mutant Arabidopsis lines which are not amenable to gas exchange measurements due to low biomass and limited leaf surface area. It is suitable for screening large numbers of replicates simultaneously as well as post-hoc analysis of previously labeled plant tissue and is complementary to downstream detection of isotopic label in targeted metabolite pools.
Keywords: 2C-methyl-D-erythritol 4-phosphate pathway; Arabidopsis thaliana; Clomazone; Fosmidomycin; Isoprenoid metabolism; Isotope ratio mass spectrometry; Photosynthetic carbon assimilation; Stable isotope labeling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Araki N, Kusumi K, Masamoto K, Niwa Y, Iba K. Temperature-sensitive Arabidopsis mutant defective in 1-deoxy-d-xylulose 5-phosphate synthase within the plastid non-mevalonate pathway of isoprenoid biosynthesis. Physiol Plant. 2000;108(1):19–24.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

