The effect of bone marrow-derived mesenchymal stem cell co-transplantation with hematopoietic stem cells on liver fibrosis alleviation and survival in patients with class III β-thalassemia major
- PMID: 33781314
- PMCID: PMC8008651
- DOI: 10.1186/s13287-021-02242-8
The effect of bone marrow-derived mesenchymal stem cell co-transplantation with hematopoietic stem cells on liver fibrosis alleviation and survival in patients with class III β-thalassemia major
Abstract
Background: Hepatic fibrosis is a common complication in transfusion-dependent thalassemia patients. Data on the co-transplantation of mesenchymal stem cells (MSCs) with hematopoietic stem cells (HSCs) in beta-thalassemia major patients are scarce. Therefore, we aimed to evaluate the effect of co-transplantation of bone marrow-derived MSC with HSCs on the liver fibrosis alleviation and transplant outcomes in class III beta-thalassemia major.
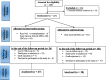
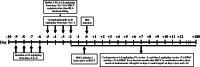
Methods: Between April 1998 and January 2017, a total of 224 consecutive patients with class III beta-thalassemia major underwent allogeneic HSCT in the Research Institute for Oncology, Hematology and Cell Therapy, Tehran University of Medical Sciences, Tehran, Iran. To assess liver fibrotic changes after transplantation, 47 patients participated in the MSC plus HSC group and 30 patients in the HSC only group at the end of the follow-up period. All patients underwent laboratory tests, especially serum ferritin and liver function testing, hepatic T2* MRI, liver biopsy, and FibroScan before and 2 years after transplantation. Kaplan-Meier curves were derived to determine survival and were compared using the log-rank test. Repeated-measure, mixed-effect linear regression models were used to examine the changes in liver fibrosis over time.
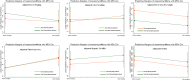
Results: The 10-year OS rate was 71.84% in the mesenchymal group and 61.89% in the non-mesenchymal group (P value = 0.294), while the 10-year TFS rate was 63.64% in the mesenchymal group and 52.78% in the non-mesenchymal group (P value = 0.285). No significant difference was observed in the 10-year NRM, rejection rate, ANC engraftment, platelet engraftment, acute GvHD, and chronic GvHD between the two groups. In addition, the results of repeated-measure, mixed-effect linear regression models showed that none of the variables determining hepatic fibrosis had a significant difference between patients receiving MSCs and patients who did not receive MSCs.
Conclusions: Based on the results of this study, a single infusion of MSCs at the time of HSCT to patients with class III beta-thalassemia major could not significantly improve the liver fibrosis alleviation and transplantation outcomes, including OS, TFS, TRM, rejection rate, ANC engraftment, platelet engraftment, acute GvHD, and chronic GvHD.
Keywords: Beta-thalassemia; Hematopoietic stem cells; Liver fibrosis; Mesenchymal stem cells; Outcome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Co-transplantation of bone marrow-derived mesenchymal stem cells with hematopoietic stem cells does not improve transplantation outcome in class III beta-thalassemia major: A prospective cohort study with long-term follow-up.Pediatr Transplant. 2021 May;25(3):e13905. doi: 10.1111/petr.13905. Epub 2020 Nov 11. Pediatr Transplant. 2021. PMID: 33179398 Clinical Trial.
-
Liver fibrosis alleviation after co-transplantation of hematopoietic stem cells with mesenchymal stem cells in patients with thalassemia major.Ann Hematol. 2018 Feb;97(2):327-334. doi: 10.1007/s00277-017-3181-9. Epub 2017 Nov 17. Ann Hematol. 2018. PMID: 29150810
-
Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Apr 20;12(1):246. doi: 10.1186/s13287-021-02304-x. Stem Cell Res Ther. 2021. PMID: 33879242 Free PMC article.
-
The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease.Stem Cell Res Ther. 2019 Jun 21;10(1):182. doi: 10.1186/s13287-019-1287-9. Stem Cell Res Ther. 2019. PMID: 31227011 Free PMC article. Review.
-
Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients.Biol Blood Marrow Transplant. 2005 May;11(5):389-98. doi: 10.1016/j.bbmt.2005.02.001. Biol Blood Marrow Transplant. 2005. PMID: 15846293 Clinical Trial.
Cited by
-
Exploring the bone marrow micro environment in thalassemia patients: potential therapeutic alternatives.Front Immunol. 2024 Aug 5;15:1403458. doi: 10.3389/fimmu.2024.1403458. eCollection 2024. Front Immunol. 2024. PMID: 39161767 Free PMC article. Review.
-
Targeting Reprogrammed Cancer-Associated Fibroblasts with Engineered Mesenchymal Stem Cell Extracellular Vesicles for Pancreatic Cancer Treatment.Biomater Res. 2024 Aug 2;28:0050. doi: 10.34133/bmr.0050. eCollection 2024. Biomater Res. 2024. PMID: 39099892 Free PMC article.
-
Stem cells for treatment of liver fibrosis/cirrhosis: clinical progress and therapeutic potential.Stem Cell Res Ther. 2022 Jul 26;13(1):356. doi: 10.1186/s13287-022-03041-5. Stem Cell Res Ther. 2022. PMID: 35883127 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

