Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage
- PMID: 33781385
- PMCID: PMC8009677
- DOI: 10.7554/eLife.60361
Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage
Abstract
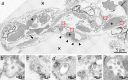
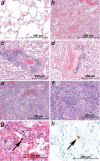
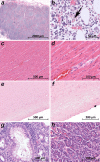
Clinical observations indicate that COVID-19 is a systemic disease. An investigation of the viral distribution within the human body and its correlation with tissue damage can aid in understanding the pathophysiology of SARS-CoV-2 infection. We present a detailed mapping of the viral RNA in 61 tissues and organs of 11 deceased patients with COVID-19. The autopsies were performed within the early postmortem interval (between 1.5 and 15 hr, mean: 5.6 hr) to minimize the bias due to viral RNA and tissue degradation. Very high viral loads (>104copies/ml) were detected in most patients' lungs, and the presence of intact viral particles in the lung tissue could be verified by transmission electron microscopy. Interestingly, viral RNA was detected throughout various extrapulmonary tissues and organs without visible tissue damage. The dissemination of SARS-CoV-2-RNA throughout the body supports the hypothesis that there is a maladaptive host response with viremia and multiorgan dysfunction.
Keywords: COVID-19; SARS-CoV-2; autopsy; early post-mortem interval; histology; immunology; infectious disease; inflammation; microbiology; transmission electron microscopy; virus.
Plain language summary
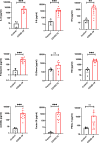
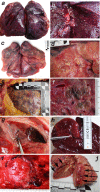
Since the discovery of the new coronavirus that causes COVID-19, scientists have been scrambling to understand the different features of the virus. While a lot more is now known about SARS-CoV-2, several key questions have proved more difficult to answer. For example, it remained unclear where the virus travels to in the body and causes the most harm. To help answer this question, Deinhardt-Emmer, Wittschieber et al. performed postmortem examinations on 11 patients who had recently died of COVID-19. After sampling 61 different organs and tissues from each patient, several tests were used to detect traces of SARS-CoV-2. The experiments showed that the largest pool of SARS-CoV-2 was present in the lungs, where it had caused severe damage to the alveolae, the delicate air sacs at the end of the lungs’ main air tubes. Small amounts of the virus were also detected in other organs and tissues, but no severe tissue damage was seen. In addition, Deinhardt-Emmer, Wittschieber et al. found that each patient had increased levels of some of the proteins involved in inflammation and blood clotting circulating their bloodstream. This suggests that the inflammation caused by SARS-CoV-2 leads to an excessive immune reaction throughout the entire body. This research provides important new insights into which areas of the body are most impacted by SARS-CoV-2. These findings may help to design more effective drug treatments that target the places SARS-CoV-2 is most likely to accumulate and help patients fight off the infection at these regions.
© 2021, Deinhardt-Emmer et al.
Conflict of interest statement
SD, DW, JS, SK, SE, KH, VV, CH, JR, AH, CE, MB, MP, NG, SN, BL, GM No competing interests declared
Figures




References
-
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New England Journal of Medicine. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

