Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan
- PMID: 33781913
- PMCID: PMC8261165
- DOI: 10.1016/j.ymthe.2021.03.020
Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan
Abstract
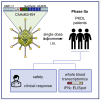
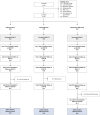
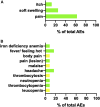
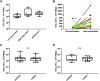
Post-kala-azar dermal leishmaniasis (PKDL) is a chronic, stigmatizing skin condition occurring frequently after apparent clinical cure from visceral leishmaniasis. Given an urgent need for new treatments, we conducted a phase IIa safety and immunogenicity trial of ChAd63-KH vaccine in Sudanese patients with persistent PKDL. LEISH2a (ClinicalTrials.gov: NCT02894008) was an open-label three-phase clinical trial involving sixteen adult and eight adolescent patients with persistent PKDL (median duration, 30 months; range, 6-180 months). Patients received a single intramuscular vaccination of 1 × 1010 viral particles (v.p.; adults only) or 7.5 × 1010 v.p. (adults and adolescents), with primary (safety) and secondary (clinical response and immunogenicity) endpoints evaluated over 42-120 days follow-up. AmBisome was provided to patients with significant remaining disease at their last visit. ChAd63-KH vaccine showed minimal adverse reactions in PKDL patients and induced potent innate and cell-mediated immune responses measured by whole-blood transcriptomics and ELISpot. 7/23 patients (30.4%) monitored to study completion showed >90% clinical improvement, and 5/23 (21.7%) showed partial improvement. A logistic regression model applied to blood transcriptomic data identified immune modules predictive of patients with >90% clinical improvement. A randomized controlled trial to determine whether these clinical responses were vaccine-related and whether ChAd63-KH vaccine has clinical utility is underway.
Keywords: ChAd63-KH vaccine; PKDL; Sudan; clinical trial; immunogenicity; leishmaniasis; safety; transcriptomics.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.J.N.L., P.M.K., and T.A. are co-authors of a patent protecting the gene insert used in candidate vaccine ChAd63-KH (Europe 10719953.1; India 315101). The authors otherwise declare no competing interests.
Figures


References
-
- World Health Organisation . 2019. Leishmaniasis.https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis
-
- Zijlstra E.E., Musa A.M., Khalil E.A., el-Hassan I.M., el-Hassan A.M. Post-kala-azar dermal leishmaniasis. Lancet Infect. Dis. 2003;3:87–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

