Discovery of a Novel Respiratory Syncytial Virus Replication Inhibitor
- PMID: 33782012
- PMCID: PMC8316115
- DOI: 10.1128/AAC.02576-20
Discovery of a Novel Respiratory Syncytial Virus Replication Inhibitor
Abstract
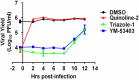
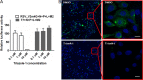
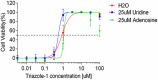
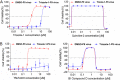
A high-throughput screen of a Roche internal chemical library based on inhibition of the respiratory syncytial virus (RSV)-induced cytopathic effect (CPE) on HEp-2 cells was performed to identify RSV inhibitors. Over 2,000 hits were identified and confirmed to be efficacious against RSV infection in vitro Here, we report the discovery of a triazole-oxadiazole derivative, designated triazole-1, as an RSV replication inhibitor, and we characterize its mechanism of action. Triazole-1 inhibited the replication of both RSV A and B subtypes with 50% inhibitory concentration (IC50) values of approximately 1 μM, but it was not effective against other viruses, including influenza virus A, human enterovirus 71 (EV71), and vaccinia virus. Triazole-1 was shown to inhibit RSV replication when added at up to 8 h after viral entry, suggesting that it inhibits RSV after viral entry. In a minigenome reporter assay in which RSV transcription regulatory sequences flanking a luciferase gene were cotransfected with RSV N/P/L/M2-1 genes into HEp-2 cells, triazole-1 demonstrated specific and dose-dependent RSV transcription inhibitory effects. Consistent with these findings, deep sequencing of the genomes of triazole-1-resistant mutants revealed a single point mutation (A to G) at nucleotide 13546 of the RSV genome, leading to a T-to-A change at amino acid position 1684 of the L protein, which is the RSV RNA polymerase for both viral transcription and replication. The effect of triazole-1 on minigenome transcription, which was mediated by the L protein containing the T1684A mutation, was significantly reduced, suggesting that the T1684A mutation alone conferred viral resistance to triazole-1.
Keywords: inhibitor; replication; respiratory syncytial virus.
Copyright © 2021 Wang et al.
Figures


Similar articles
-
Preclinical Characterization of PC786, an Inhaled Small-Molecule Respiratory Syncytial Virus L Protein Polymerase Inhibitor.Antimicrob Agents Chemother. 2017 Aug 24;61(9):e00737-17. doi: 10.1128/AAC.00737-17. Print 2017 Sep. Antimicrob Agents Chemother. 2017. PMID: 28652242 Free PMC article.
-
Cyclopiazonic acid, an inhibitor of calcium-dependent ATPases with antiviral activity against human respiratory syncytial virus.Antiviral Res. 2016 Aug;132:38-45. doi: 10.1016/j.antiviral.2016.05.010. Epub 2016 May 17. Antiviral Res. 2016. PMID: 27210812
-
Respiratory Syncytial Virus Inhibitor AZ-27 Differentially Inhibits Different Polymerase Activities at the Promoter.J Virol. 2015 Aug;89(15):7786-98. doi: 10.1128/JVI.00530-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995255 Free PMC article.
-
Respiratory Syncytial Virus Phosphoprotein Residue S156 Plays a Role in Regulating Genome Transcription and Replication.J Virol. 2021 Nov 23;95(24):e0120621. doi: 10.1128/JVI.01206-21. Epub 2021 Oct 6. J Virol. 2021. PMID: 34613802 Free PMC article.
-
Structural Insights into the Respiratory Syncytial Virus RNA Synthesis Complexes.Viruses. 2021 May 5;13(5):834. doi: 10.3390/v13050834. Viruses. 2021. PMID: 34063087 Free PMC article. Review.
Cited by
-
Structural basis of paramyxo- and pneumovirus polymerase inhibition by non-nucleoside small-molecule antivirals.Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0080024. doi: 10.1128/aac.00800-24. Epub 2024 Aug 20. Antimicrob Agents Chemother. 2024. PMID: 39162479 Free PMC article. Review.
-
Computational Evidence for Bisartan Arginine Blockers as Next-Generation Pan-Antiviral Therapeutics Targeting SARS-CoV-2, Influenza, and Respiratory Syncytial Viruses.Viruses. 2024 Nov 14;16(11):1776. doi: 10.3390/v16111776. Viruses. 2024. PMID: 39599890 Free PMC article.
-
Exploring the secrets of virus entry: the first respiratory syncytial virus carrying beta lactamase.Front Microbiol. 2024 Feb 16;15:1339569. doi: 10.3389/fmicb.2024.1339569. eCollection 2024. Front Microbiol. 2024. PMID: 38455070 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

