Ramipril and Cardiovascular Outcomes in Patients on Maintenance Hemodialysis: The ARCADIA Multicenter Randomized Controlled Trial
- PMID: 33782036
- PMCID: PMC8092055
- DOI: 10.2215/CJN.12940820
Ramipril and Cardiovascular Outcomes in Patients on Maintenance Hemodialysis: The ARCADIA Multicenter Randomized Controlled Trial
Abstract
Background and objectives: Renin-angiotensin system (RAS) inhibitors reduce cardiovascular morbidity and mortality in patients with CKD. We evaluated the cardioprotective effects of the angiotensin-converting enzyme inhibitor ramipril in patients on maintenance hemodialysis.
Design, setting, participants, & measurements: In this phase 3, prospective, randomized, open-label, blinded end point, parallel, multicenter trial, we recruited patients on maintenance hemodialysis with hypertension and/or left ventricular hypertrophy from 28 Italian centers. Between July 2009 and February 2014, 140 participants were randomized to ramipril (1.25-10 mg/d) and 129 participants were allocated to non-RAS inhibition therapy, both titrated up to the maximally tolerated dose to achieve predefined target BP values. The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. Secondary end points included the single components of the primary end point, new-onset or recurrence of atrial fibrillation, hospitalizations for symptomatic fluid overload, thrombosis or stenosis of the arteriovenous fistula, and changes in cardiac mass index. All outcomes were evaluated up to 42 months after randomization.
Results: At comparable BP control, 23 participants on ramipril (16%) and 24 on non-RAS inhibitor therapy (19%) reached the primary composite end point (hazard ratio, 0.93; 95% confidence interval, 0.52 to 1.64; P=0.80). Ramipril reduced cardiac mass index at 1 year of follow-up (between-group difference in change from baseline: -16.3 g/m2; 95% confidence interval, -29.4 to -3.1), but did not significantly affect the other secondary outcomes. Hypotensive episodes were more frequent in participants allocated to ramipril than controls (41% versus 12%). Twenty participants on ramipril and nine controls developed cancer, including six gastrointestinal malignancies on ramipril (four were fatal), compared with none in controls.
Conclusions: Ramipril did not reduce the risk of major cardiovascular events in patients on maintenance hemodialysis.
Clinical trial registry name and registration number: ARCADIA, NCT00985322 and European Union Drug Regulating Authorities Clinical Trials Database number 2008-003529-17.
Keywords: ACE inhibitors; Ramipril; cardiovascular events; hemodialysis; renin angiotensin system.
Copyright © 2021 by the American Society of Nephrology.
Figures


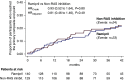
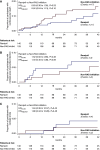

Comment in
-
Therapeutic Options to Improve Cardiovascular Outcomes with Long-Term Hemodialysis.Clin J Am Soc Nephrol. 2021 Apr 7;16(4):511-513. doi: 10.2215/CJN.02010221. Epub 2021 Mar 29. Clin J Am Soc Nephrol. 2021. PMID: 33782038 Free PMC article. No abstract available.
References
-
- Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg-Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, Gaipov A, Gillen D, Gipson D, Gu H, Guro P, Haggerty D, Han Y, He K, Herman W, Heung M, Hirth RA, Hsiung JT, Hutton D, Inoue A, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kleine CE, Kovesdy CP, Krueter W, Kurtz V, Li Y, Liu S, Marroquin MV, McCullough K, Molnar MZ, Modi Z, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O’Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Repeck K, Rhee CM, Schaubel DE, Schrager J, Selewski DT, Shamraj R, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Kurella Tamura M, Tilea A, Turf M, Wang D, Weng W, Woodside KJ, Wyncott A, Xiang J, Xin X, Yin M, You AS, Zhang X, Zhou H, Shahinian V: US Renal Data System 2018 annual data report: Epidemiology of kidney disease in the United States. Am J Kidney Dis 73[Suppl 1]: A7–A8, 2019 - PMC - PubMed
-
- Zannad F, Kessler M, Lehert P, Grünfeld JP, Thuilliez C, Leizorovicz A, Lechat P: Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70: 1318–1324, 2006 - PubMed
-
- Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 - PubMed
-
- Jungers P, Nguyen Khoa T, Massy ZA, Zingraff J, Labrunie M, Descamps-Latscha B, Man NK: Incidence of atherosclerotic arterial occlusive accidents in predialysis and dialysis patients: A multicentric study in the Ile de France district. Nephrol Dial Transplant 14: 898–902, 1999 - PubMed
-
- Brown JH, Hunt LP, Vites NP, Short CD, Gokal R, Mallick NP: Comparative mortality from cardiovascular disease in patients with chronic renal failure. Nephrol Dial Transplant 9: 1136–1142, 1994 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

