Dynamic extrinsic pacing of the HOX clock in human axial progenitors controls motor neuron subtype specification
- PMID: 33782043
- PMCID: PMC8034877
- DOI: 10.1242/dev.194514
Dynamic extrinsic pacing of the HOX clock in human axial progenitors controls motor neuron subtype specification
Abstract
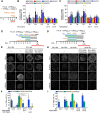
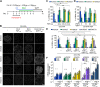
Rostro-caudal patterning of vertebrates depends on the temporally progressive activation of HOX genes within axial stem cells that fuel axial embryo elongation. Whether the pace of sequential activation of HOX genes, the 'HOX clock', is controlled by intrinsic chromatin-based timing mechanisms or by temporal changes in extrinsic cues remains unclear. Here, we studied HOX clock pacing in human pluripotent stem cell-derived axial progenitors differentiating into diverse spinal cord motor neuron subtypes. We show that the progressive activation of caudal HOX genes is controlled by a dynamic increase in FGF signaling. Blocking the FGF pathway stalled induction of HOX genes, while a precocious increase of FGF, alone or with GDF11 ligand, accelerated the HOX clock. Cells differentiated under accelerated HOX induction generated appropriate posterior motor neuron subtypes found along the human embryonic spinal cord. The pacing of the HOX clock is thus dynamically regulated by exposure to secreted cues. Its manipulation by extrinsic factors provides synchronized access to multiple human neuronal subtypes of distinct rostro-caudal identities for basic and translational applications.This article has an associated 'The people behind the papers' interview.
Keywords: Axial; HOX genes; Human; Motor neurons; Pluripotent stem cells; Spinal cord.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Amoroso, M. W., Croft, G. F., Williams, D. J., O'Keeffe, S., Carrasco, M. A., Davis, A. R., Roybon, L., Oakley, D. H., Maniatis, T., Henderson, C. E.et al. (2013). Accelerated high-yield generation of limb-innervating motor neurons from human stem cells. J. Neurosci. 33, 574-586. 10.1523/JNEUROSCI.0906-12.2013 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

