Testicular Sertoli cell tumour and potentially testicular Leydig cell tumour are features of DICER1 syndrome
- PMID: 33782093
- PMCID: PMC9743800
- DOI: 10.1136/jmedgenet-2020-107434
Testicular Sertoli cell tumour and potentially testicular Leydig cell tumour are features of DICER1 syndrome
Abstract
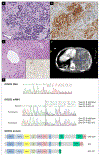
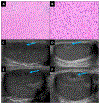
DICER1 syndrome is a rare paediatric autosomal dominant inherited disorder predisposing to various benign and malignant tumours. It is caused by a germline pathogenic variant in DICER1, and the second hit for tumour development is usually a missense hotspot pathogenic variant in the DICER1 ribonuclease IIIb domain. While DICER1 predisposing variants account for about 60% of ovarian Sertoli-Leydig cell tumours, no DICER1-related testicular stromal tumours have been described. Here we report the first two cases of testicular stromal tumours in children carrying a DICER1 germline pathogenic variant: a case of Sertoli cell tumour and a case of Leydig cell tumour diagnosed at 2 and 12 years of age, respectively. A somatic DICER1 hotspot pathogenic variant was detected in the Sertoli cell tumour. This report extends the spectrum of DICER1-related tumours to include testicular Sertoli cell tumour and potentially testicular Leydig cell tumour. Diagnosis of a testicular Sertoli cell tumour should prompt DICER1 genetic testing so that patients with a DICER1 germline pathogenic variant can benefit from established surveillance guidelines. DICER1 genetic evaluation may be considered for testicular Leydig cell tumour. Our findings suggest that miRNA dysregulation underlies the aetiology of some testicular stromal tumours.
Keywords: genetic predisposition to disease; medical oncology; paediatrics.
© Author(s) (or their employer(s)) 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Kock L, Wu MK, Foulkes WD. Ten years of DICER1 mutations: Provenance, distribution, and associated phenotypes. Hum. Mutat 2019;40:1939–1953. - PubMed
-
- Schultz KAP, Harris AK, Finch M, Dehner LP, Brown JB, Gershenson DM, Young RH, Field A, Yu W, Turner J, Cost NG, Schneider DT, Stewart DR, Frazier AL, Messinger Y, Hill DA. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: Clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol. Oncol 2017;147:521–527. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
