Cancer immunotherapy in special challenging populations: recommendations of the Advisory Committee of Spanish Melanoma Group (GEM)
- PMID: 33782108
- PMCID: PMC8009216
- DOI: 10.1136/jitc-2020-001664
Cancer immunotherapy in special challenging populations: recommendations of the Advisory Committee of Spanish Melanoma Group (GEM)
Erratum in
-
Correction: Cancer immunotherapy in special challenging populations: recommendations of the advisory Committee of Spanish Melanoma Group (GEM).J Immunother Cancer. 2022 Feb;10(2):e001664corr1. doi: 10.1136/jitc-2020-001664corr1. J Immunother Cancer. 2022. PMID: 35177416 Free PMC article. No abstract available.
Abstract
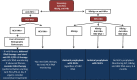
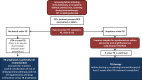
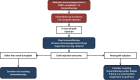
Cancer immunotherapy based on the use of antibodies targeting the so-called checkpoint inhibitors, such as programmed cell death-1 receptor, its ligand, or CTLA-4, has shown durable clinical benefit and survival improvement in melanoma and other tumors. However, there are some special situations that could be a challenge for clinical management. Persons with chronic infections, such as HIV-1 or viral hepatitis, latent tuberculosis, or a history of solid organ transplantation, could be candidates for cancer immunotherapy, but their management requires a multidisciplinary approach. The Spanish Melanoma Group (GEM) panel in collaboration with experts in virology and immunology from different centers in Spain reviewed the literature and developed evidence-based guidelines for cancer immunotherapy management in patients with chronic infections and immunosuppression. These are the first clinical guidelines for cancer immunotherapy treatment in special challenging populations. Cancer immunotherapy in chronically infected or immunosuppressed patients is feasible but needs a multidisciplinary approach in order to decrease the risk of complications related to the coexistent comorbidities.
Keywords: immunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
