Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles
- PMID: 33782168
- PMCID: PMC8259679
- DOI: 10.1681/ASN.2020081142
Comparing Approaches to Normalize, Quantify, and Characterize Urinary Extracellular Vesicles
Abstract
Background: Urinary extracellular vesicles (uEVs) are a promising source for biomarker discovery, but optimal approaches for normalization, quantification, and characterization in spot urines are unclear.
Methods: Urine samples were analyzed in a water-loading study, from healthy subjects and patients with kidney disease. Urine particles were quantified in whole urine using nanoparticle tracking analysis (NTA), time-resolved fluorescence immunoassay (TR-FIA), and EVQuant, a novel method quantifying particles via gel immobilization.
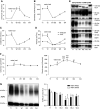
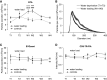
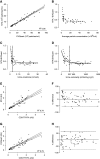
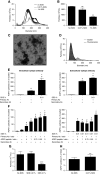
Results: Urine particle and creatinine concentrations were highly correlated in the water-loading study (R2 0.96) and in random spot urines from healthy subjects (R2 0.47-0.95) and patients (R2 0.41-0.81). Water loading reduced aquaporin-2 but increased Tamm-Horsfall protein (THP) and particle detection by NTA. This finding was attributed to hypotonicity increasing uEV size (more EVs reach the NTA size detection limit) and reducing THP polymerization. Adding THP to urine also significantly increased particle count by NTA. In both fluorescence NTA and EVQuant, adding 0.01% SDS maintained uEV integrity and increased aquaporin-2 detection. Comparison of intracellular- and extracellular-epitope antibodies suggested the presence of reverse topology uEVs. The exosome markers CD9 and CD63 colocalized and immunoprecipitated selectively with distal nephron markers. Conclusions uEV concentration is highly correlated with urine creatinine, potentially replacing the need for uEV quantification to normalize spot urines. Additional findings relevant for future uEV studies in whole urine include the interference of THP with NTA, excretion of larger uEVs in dilute urine, the ability to use detergent to increase intracellular-epitope recognition in uEVs, and CD9 or CD63 capture of nephron segment-specific EVs.
Keywords: aquaporin-2; biomarker; creatinine; exosomes; normalization; osmolality; particles; tetraspanin; urinary extracellular vesicles; uromodulin.
Copyright © 2021 by the American Society of Nephrology.
Figures





Comment in
-
Urinary Vesicles: Are They Ready for Real-World Use?J Am Soc Nephrol. 2021 May 3;32(5):1013-1015. doi: 10.1681/ASN.2021030332. Epub 2021 Mar 29. J Am Soc Nephrol. 2021. PMID: 33782170 Free PMC article. No abstract available.
References
-
- Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

