Activity of docetaxel, carboplatin, and doxorubicin in patient-derived triple-negative breast cancer xenografts
- PMID: 33782404
- PMCID: PMC8007714
- DOI: 10.1038/s41598-021-85962-4
Activity of docetaxel, carboplatin, and doxorubicin in patient-derived triple-negative breast cancer xenografts
Abstract
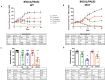
Triple-negative breast cancer (TNBC) is highly responsive to neoadjuvant polychemotherapy regimens including anthracyclines, taxanes, and, more recently, carboplatin. However, there is inadequate information on the individual contribution of each of these agents to the global activity of the combinations, and the use of combinations of up to four of these drugs is associated with relevant toxicity. Identifying single-drug activity in the clinical neoadjuvant setting is challenging. We developed patient-derived xenografts (PDXs) from several chemotherapy-naïve TNBC samples to assess the antitumor activity of single drugs and combinations of drugs. PDXs were established from chemotherapy-naïve TNBC samples. Nine TNBC PDX models (all of which corresponded to a basal-like phenotype according to the PAM50 classifier) were treated with carboplatin, docetaxel, and doxorubicin and the combination of docetaxel and carboplatin. Only one of nine PDX models showed sensitivity to doxorubicin, while eight of nine PDX models showed sensitivity to docetaxel and carboplatin as single agents. The 3 PDX models derived from patients with gBRCA-1 or gPALB2 mutations were very sensitive to carboplatin single agent. All 6 PDX models from patients without hereditary germ-line mutations showed increased sensitivity to the combination of docetaxel and carboplatin. In the present study, docetaxel and carboplatin single agents were active drugs against basal-like TNBC, while doxorubicin monotherapy showed low activity. The combination of docetaxel and carboplatin was more effective than the drugs used as single agents, except in the PDX from patients with gBRCA1/PALB2 mutations.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Cleere DW. Triple-negative breast cancer: a clinical update. Commun. Oncol. 2010;7(5):203–211. doi: 10.1016/S1548-5315(11)70394-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

