Severity of heterosubtypic influenza virus infection in ferrets is reduced by live attenuated influenza vaccine
- PMID: 33782409
- PMCID: PMC8007727
- DOI: 10.1038/s41541-021-00306-7
Severity of heterosubtypic influenza virus infection in ferrets is reduced by live attenuated influenza vaccine
Abstract
Live attenuated influenza vaccine (LAIV) is widely used to protect humans from seasonal influenza infection, particularly in children. In contrast to inactivated vaccines, the LAIV can induce both mucosal and cellular immune responses. Here we show that a single dose of monovalent H1N1pdm09-specific LAIV in the ferret model is fully protective against a subsequent wild-type H1N1pdm09 challenge, and furthermore reduces the severity of disease following challenge with a different influenza A subtype (H3N2). The reduced severity comprised reductions in weight loss and fever, as well as more rapid clearance of virus, compared to non-vaccinated H3N2-challenged ferrets. No H3N2-neutralizing antibodies were detected in vaccinated ferret sera. Rather, heterosubtypic protection correlated with interferon-gamma+ (IFN-γ+) T-cell responses measured in peripheral blood and in lung lymphocytes. The IFN-γ+ cells were cross-reactive to H3N2 virus even when obtained from vaccinated animals that had never been exposed to H3N2 virus. We believe this study provides compelling evidence that the LAIV can provide a significant reduction in infection and symptoms when challenged with heterosubtypic influenza strains not included in the LAIV, highlighting the importance of cross-reactive T-cells in the design of a universal influenza vaccine.
Conflict of interest statement
At the time of manuscript preparation, O.D. and H.B. were employees and shareholders of AstraZeneca plc. AstraZeneca plc are the manufacturers of Fluenz® Tetra/FluMist® Quadrivalent intranasal influenza live virus vaccine. The authors declare that there are no other competing interests.
Figures
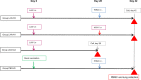
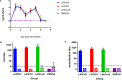

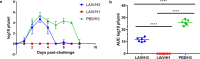




References
LinkOut - more resources
Full Text Sources
Other Literature Sources

