Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma
- PMID: 33782460
- PMCID: PMC8007640
- DOI: 10.1038/s41598-021-86391-z
Camel whey protein hydrolysates induced G2/M cellcycle arrest in human colorectal carcinoma
Abstract
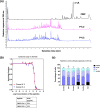
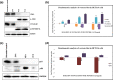
Camel milk has been gaining immmense importance due to high nutritious value and medicinal properties. Peptides from milk proteins is gaining popularity in various therapeutics including human cancer. The study was aimed to investigate the anti-cancerous and anti-inflammatory properties of camel whey protein hydrolysates (CWPHs). CWPHs were generated at three temperatures (30 ℃, 37 ℃, and 45 ℃), two hydrolysis timepoints (120 and 360 min) and with three different enzyme concentrations (0.5, 1 and 2 %). CWPHs demonstrated an increase in anti-inflammatory effect between 732.50 (P-6.1) and 3779.16 (P-2.1) µg Dicolfenac Sodium Equivalent (DSE)/mg protein. CWPHs (P-4.3 & 5.2) inhibited growth of human colon carcinoma cells (HCT116) with an IC50 value of 231 and 221 μg/ml, respectively. P-4.3 induced G2/M cell cycle arrest and modulated the expression of Cdk1, p-Cdk1, Cyclin B1, p-histone H3, p21 and p53. Docking of two peptides (AHLEQVLLR and ALPNIDPPTVER) from CWPHs (P-4.3) identified Polo like kinase 1 as a potential target, which strongly supports our in vitro data and provides an encouraging insight into developing a novel peptide-based anticancer formulation. These results suggest that the active component, CWPHs (P-4.3), can be further studied and modeled to form a small molecule anti-cancerous therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

