The gut microbiota metabolite urolithin A inhibits NF-κB activation in LPS stimulated BMDMs
- PMID: 33782464
- PMCID: PMC8007722
- DOI: 10.1038/s41598-021-86514-6
The gut microbiota metabolite urolithin A inhibits NF-κB activation in LPS stimulated BMDMs
Abstract
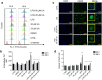
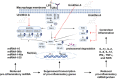
Inflammation is a natural defense process of the innate immune system, associated with the release of proinflammatory cytokines such as interleukin-1β, interleukin-6, interleukin-12 and TNFα; and enzymes including iNOS through the activation and nuclear translocation of NF-κB p65 due to the phosphorylation of IκBα. Regulation of intracellular Ca2+ is considered a promising strategy for the prevention of reactive oxygen species (ROS) production and accumulation of DNA double strand breaks (DSBs) that occurs in inflammatory-associated-diseases. Among the metabolites of ellagitannins that are produced in the gut microbiome, urolithin A (UA) has received an increasing attention as a novel candidate with anti-inflammatory and anti-oxidant effects. Here, we investigated the effect of UA on the suppression of pro-inflammatory molecules and NF-κB activation by targeting TLR4 signalling pathway. We also identified the influence of UA on Ca2+ entry, ROS production and DSBs availability in murine bone-marrow-derived macrophages challenged with lipopolysaccharides (LPS). We found that UA inhibits IκBα phosphorylation and supresses MAPK and PI3K activation. In addition, UA was able to reduce calcium entry, ROS production and DSBs availability. In conclusion, we suggest that urolithin A is a promising therapeutic agent for treating inflammatory diseases through suppression of NF-κB and preserving DNA through maintaining intracellular calcium and ROS homeostasis.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

