Sensitive quantification of carbon monoxide in vivo reveals a protective role of circulating hemoglobin in CO intoxication
- PMID: 33782534
- PMCID: PMC8007703
- DOI: 10.1038/s42003-021-01880-1
Sensitive quantification of carbon monoxide in vivo reveals a protective role of circulating hemoglobin in CO intoxication
Abstract
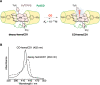
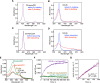
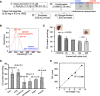
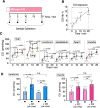
Carbon monoxide (CO) is a gaseous molecule known as the silent killer. It is widely believed that an increase in blood carboxyhemoglobin (CO-Hb) is the best biomarker to define CO intoxication, while the fact that CO accumulation in tissues is the most likely direct cause of mortality is less investigated. There is no reliable method other than gas chromatography to accurately determine CO content in tissues. Here we report the properties and usage of hemoCD1, a synthetic supramolecular compound composed of an iron(II)porphyrin and a cyclodextrin dimer, as an accessible reagent for a simple colorimetric assay to quantify CO in biological samples. The assay was validated in various organ tissues collected from rats under normal conditions and after exposure to CO. The kinetic profile of CO in blood and tissues after CO treatment suggested that CO accumulation in tissues is prevented by circulating Hb, revealing a protective role of Hb in CO intoxication. Furthermore, hemoCD1 was used in vivo as a CO removal agent, showing that it acts as an effective adjuvant to O2 ventilation to eliminate residual CO accumulated in organs, including the brain. These findings open new therapeutic perspectives to counteract the toxicity associated with CO poisoning.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dydek, T. M. Investigating carbon monoxide poisonings. In Carbon Monoxide Poisoning (ed. Penney, D. G.) Ch. 12 (CRC and Taylor and Francis Press, 2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

