PXR mediates mifepristone-induced hepatomegaly in mice
- PMID: 33782543
- PMCID: PMC8724318
- DOI: 10.1038/s41401-021-00633-4
PXR mediates mifepristone-induced hepatomegaly in mice
Abstract
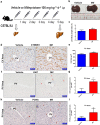
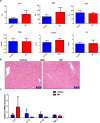
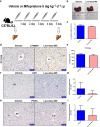
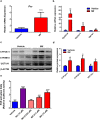
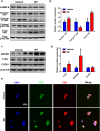
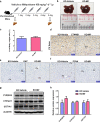
Mifepristone (Mif), an effective synthetic steroidal antiprogesterone drug, is widely used for medical abortion and pregnancy prevention. Due to its anti-glucocorticoid effect, high-dose Mif is also used to treat Cushing's syndrome. Mif was reported to active pregnane X receptor (PXR) in vitro and PXR can induce hepatomegaly via activation and interaction with yes-associated protein (YAP) pathway. High-dose Mif was reported to induce hepatomegaly in rats and mice, but the underlying mechanism remains unclear. Here, the role of PXR was studied in Mif-induced hepatomegaly in C57BL/6 mice and Pxr-knockout mice. The results demonstrated that high-dose Mif (100 mg · kg-1 · d-1, i.p.) treatment for 5 days significantly induced hepatomegaly with enlarged hepatocytes and promoted proliferation, but low dose of Mif (5 mg · kg-1 · d-1, i.p.) cannot induce hepatomegaly. The dual-luciferase reporter gene assays showed that Mif can activate human PXR in a concentration-dependent manner. In addition, Mif could promote nuclear translocation of PXR and YAP, and significantly induced the expression of PXR, YAP, and their target proteins such as CYP3A11, CYP2B10, UGT1A1, ANKRD, and CTGF. However, Mif (100 mg · kg-1 · d-1, i.p.) failed to induce hepatomegaly in Pxr-knockout mice, as well as hepatocyte enlargement and proliferation, further indicating that Mif-induced hepatomegaly is PXR-dependent. In summary, this study demonstrated that PXR-mediated Mif-induced hepatomegaly in mice probably via activation of YAP pathway. This study provides new insights in Mif-induced hepatomegaly, and provides novel evidence on the crucial function of PXR in liver enlargement and regeneration.
Keywords: hepatomegaly; mifepristone; pregnane X receptor (PXR); yes-associated protein (YAP).
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Ulmann A, Teutsch G, Philibert D. RU 486. Sci Am. 1990;262:42–8. - PubMed
-
- Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril. 1991;56:385–93. - PubMed
-
- Chen J, Wang J, Shao J, Gao Y, Xu J, Yu S, et al. The unique pharmacological characteristics of mifepristone (RU486): from terminating pregnancy to preventing cancer metastasis. Med Res Rev. 2014;34:979–1000. - PubMed
-
- Bamberger CM, Chrousos GP. The glucocorticoid receptor and RU 486 in man. Ann N Y Acad Sci. 1995;761:296–310. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

