Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice
- PMID: 33782605
- PMCID: PMC8245161
- DOI: 10.1038/s41588-021-00803-4
Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice
Erratum in
-
Author Correction: Gain-of-function variants in SYK cause immune dysregulation and systemic inflammation in humans and mice.Nat Genet. 2022 Feb;54(2):213. doi: 10.1038/s41588-021-00998-6. Nat Genet. 2022. PMID: 34987219 No abstract available.
Abstract
Spleen tyrosine kinase (SYK) is a critical immune signaling molecule and therapeutic target. We identified damaging monoallelic SYK variants in six patients with immune deficiency, multi-organ inflammatory disease such as colitis, arthritis and dermatitis, and diffuse large B cell lymphomas. The SYK variants increased phosphorylation and enhanced downstream signaling, indicating gain of function. A knock-in (SYK-Ser544Tyr) mouse model of a patient variant (p.Ser550Tyr) recapitulated aspects of the human disease that could be partially treated with a SYK inhibitor or transplantation of bone marrow from wild-type mice. Our studies demonstrate that SYK gain-of-function variants result in a potentially treatable form of inflammatory disease.
Figures
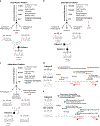






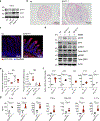

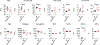
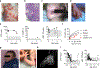


Comment in
-
The sickening consequences of too much SYK signaling.Nat Genet. 2021 Apr;53(4):432-434. doi: 10.1038/s41588-021-00837-8. Nat Genet. 2021. PMID: 33782606 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

