Identification and characterization of novel candidate compounds targeting 6- and 7-transmembrane μ-opioid receptor isoforms
- PMID: 33782947
- PMCID: PMC10697213
- DOI: 10.1111/bph.15463
Identification and characterization of novel candidate compounds targeting 6- and 7-transmembrane μ-opioid receptor isoforms
Abstract
Background and purpose: The μ-opioid receptor (μ receptor) is the primary target for opioid analgesics. The 7-transmembrane (TM) and 6TM μ receptor isoforms mediate inhibitory and excitatory cellular effects. Here, we developed compounds selective for 6TM- or 7TM-μ receptors to further our understanding of the pharmacodynamic properties of μ receptors.
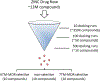
Experimental approach: We performed virtual screening of the ZINC Drug Now library of compounds using in silico 7TM- and 6TM-μ receptor structural models and identified potential compounds that are selective for 6TM- and/or 7TM-μ receptors. Subsequently, we characterized the most promising candidate compounds in functional in vitro studies using Be2C neuroblastoma transfected cells, behavioural in vivo pain assays using various knockout mice and in ex vivo electrophysiology studies.
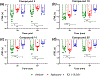
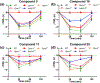
Key results: Our virtual screen identified 30 potential candidate compounds. Subsequent functional in vitro cellular assays shortlisted four compounds (#5, 10, 11 and 25) that demonstrated 6TM- or 7TM-μ receptor-dependent NO release. In in vivo pain assays these compounds also produced dose-dependent hyperalgesic responses. Studies using mice that lack specific opioid receptors further established the μ receptor-dependent nature of identified novel ligands. Ex vivo electrophysiological studies on spontaneous excitatory postsynaptic currents in isolated spinal cord slices also validated the hyperalgesic properties of the most potent 6TM- (#10) and 7TM-μ receptor (#5) ligands.
Conclusion and implications: Our novel compounds represent a new class of ligands for μ receptors and will serve as valuable research tools to facilitate the development of opioids with significant analgesic efficacy and fewer side-effects.
Keywords: 6TM-μ receptor; 7TM-μ receptor; opioid; opioid receptor isoform; pain.
© 2021 The British Pharmacological Society.
Conflict of interest statement
CONFLICT OF INTEREST
Authors have no competing interests to disclose.
Figures
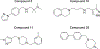




References
-
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & Collaborators C (2019). The concise guide to pharmacology 2019/20: G protein-coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 - DOI - PMC - PubMed
-
- Alexander SPH, Fabbro D, Kelly E, Mathie A, Peters JA, Veale EL, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 - DOI - PMC - PubMed
-
- Alexander SPH, Mathie A, Peters JA, Veale EL, Striessnig J, Kelly E, Armstrong JF, Faccenda E, Harding SD, Pawson AJ, Sharman JL, Southan C, Davies JA, & CGTP Collaborators. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

