Understanding the relationship between norovirus diversity and immunity
- PMID: 33783322
- PMCID: PMC8018473
- DOI: 10.1080/19490976.2021.1900994
Understanding the relationship between norovirus diversity and immunity
Abstract
Human noroviruses are the most common viral cause of acute gastroenteritis worldwide. Currently, there are no approved vaccines or specific therapeutics to treat the disease. Some obstacles delaying the development of a norovirus vaccine are: (i) the extreme diversity presented by noroviruses; (ii) our incomplete understanding of immunity to noroviruses; and (iii) the lack of a robust cell culture system or animal model for human noroviruses. Recent advances in in vitro cultivation of norovirus, novel approaches applied to viral genomics and immunity, and completion of vaccine trials and birth cohort studies have provided new information toward a better understanding of norovirus immunity. Here, we will discuss the complex relationship between norovirus diversity and correlates of protection for human noroviruses, and how this information could be used to guide the development of cross-protective vaccines.
Keywords: Norovirus; correlates of protection; gastroenteritis; immunity; vaccines.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
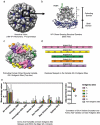
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
