First blood: the endothelial origins of hematopoietic progenitors
- PMID: 33783643
- PMCID: PMC8205888
- DOI: 10.1007/s10456-021-09783-9
First blood: the endothelial origins of hematopoietic progenitors
Abstract
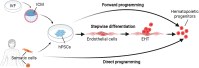
Hematopoiesis in vertebrate embryos occurs in temporally and spatially overlapping waves in close proximity to blood vascular endothelial cells. Initially, yolk sac hematopoiesis produces primitive erythrocytes, megakaryocytes, and macrophages. Thereafter, sequential waves of definitive hematopoiesis arise from yolk sac and intraembryonic hemogenic endothelia through an endothelial-to-hematopoietic transition (EHT). During EHT, the endothelial and hematopoietic transcriptional programs are tightly co-regulated to orchestrate a shift in cell identity. In the yolk sac, EHT generates erythro-myeloid progenitors, which upon migration to the liver differentiate into fetal blood cells, including erythrocytes and tissue-resident macrophages. In the dorsal aorta, EHT produces hematopoietic stem cells, which engraft the fetal liver and then the bone marrow to sustain adult hematopoiesis. Recent studies have defined the relationship between the developing vascular and hematopoietic systems in animal models, including molecular mechanisms that drive the hemato-endothelial transcription program for EHT. Moreover, human pluripotent stem cells have enabled modeling of fetal human hematopoiesis and have begun to generate cell types of clinical interest for regenerative medicine.
Keywords: EndoHT; Endothelium; Erythro-myeloid progenitor; Fetal liver; Hematopoiesis; Hematopoietic stem cell; Tissue‐resident macrophage; Yolk sac.
Conflict of interest statement
The authors have no conflicts or competing interests to report.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

