Selective killing of homologous recombination-deficient cancer cell lines by inhibitors of the RPA:RAD52 protein-protein interaction
- PMID: 33784323
- PMCID: PMC8009417
- DOI: 10.1371/journal.pone.0248941
Selective killing of homologous recombination-deficient cancer cell lines by inhibitors of the RPA:RAD52 protein-protein interaction
Abstract
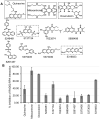
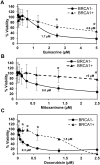
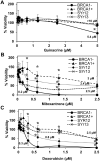
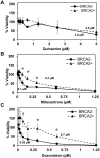
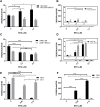
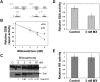
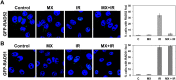
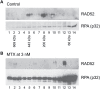
Synthetic lethality is a successful strategy employed to develop selective chemotherapeutics against cancer cells. Inactivation of RAD52 is synthetically lethal to homologous recombination (HR) deficient cancer cell lines. Replication protein A (RPA) recruits RAD52 to repair sites, and the formation of this protein-protein complex is critical for RAD52 activity. To discover small molecules that inhibit the RPA:RAD52 protein-protein interaction (PPI), we screened chemical libraries with our newly developed Fluorescence-based protein-protein Interaction Assay (FluorIA). Eleven compounds were identified, including FDA-approved drugs (quinacrine, mitoxantrone, and doxorubicin). The FluorIA was used to rank the compounds by their ability to inhibit the RPA:RAD52 PPI and showed mitoxantrone and doxorubicin to be the most effective. Initial studies using the three FDA-approved drugs showed selective killing of BRCA1-mutated breast cancer cells (HCC1937), BRCA2-mutated ovarian cancer cells (PE01), and BRCA1-mutated ovarian cancer cells (UWB1.289). It was noteworthy that selective killing was seen in cells known to be resistant to PARP inhibitors (HCC1937 and UWB1 SYr13). A cell-based double-strand break (DSB) repair assay indicated that mitoxantrone significantly suppressed RAD52-dependent single-strand annealing (SSA) and mitoxantrone treatment disrupted the RPA:RAD52 PPI in cells. Furthermore, mitoxantrone reduced radiation-induced foci-formation of RAD52 with no significant activity against RAD51 foci formation. The results indicate that the RPA:RAD52 PPI could be a therapeutic target for HR-deficient cancers. These data also suggest that RAD52 is one of the targets of mitoxantrone and related compounds.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al.. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–51. 10.1016/S0140-6736(10)60893-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

