CD148 Deficiency in Fibroblasts Promotes the Development of Pulmonary Fibrosis
- PMID: 33784491
- PMCID: PMC8513593
- DOI: 10.1164/rccm.202008-3100OC
CD148 Deficiency in Fibroblasts Promotes the Development of Pulmonary Fibrosis
Abstract
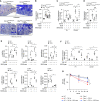
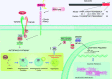
Rationale: CD148/PTRJ (receptor-like protein tyrosine phosphatase η) exerts antifibrotic effects in experimental pulmonary fibrosis via interactions with its ligand syndecan-2; however, the role of CD148 in human pulmonary fibrosis remains incompletely characterized.Objectives: We investigated the role of CD148 in the profibrotic phenotype of fibroblasts in idiopathic pulmonary fibrosis (IPF).Methods: Conditional CD148 fibroblast-specific knockout mice were generated and exposed to bleomycin and then assessed for pulmonary fibrosis. Lung fibroblasts (mouse lung and human IPF lung), and precision-cut lung slices from human patients with IPF were isolated and subjected to experimental treatments. A CD148-activating 18-aa mimetic peptide (SDC2-pep) derived from syndecan-2 was evaluated for its therapeutic potential.Measurements and Main Results: CD148 expression was downregulated in IPF lungs and fibroblasts. In human IPF lung fibroblasts, silencing of CD148 increased extracellular matrix production and resistance to apoptosis, whereas overexpression of CD148 reversed the profibrotic phenotype. CD148 fibroblast-specific knockout mice displayed increased pulmonary fibrosis after bleomycin challenge compared with control mice. CD148-deficient fibroblasts exhibited hyperactivated PI3K/Akt/mTOR signaling, reduced autophagy, and increased p62 accumulation, which induced NF-κB activation and profibrotic gene expression. SDC2-pep reduced pulmonary fibrosis in vivo and inhibited IPF-derived fibroblast activation. In precision-cut lung slices from patients with IPF and control patients, SDC2-pep attenuated profibrotic gene expression in IPF and normal lungs stimulated with profibrotic stimuli.Conclusions: Lung fibroblast CD148 activation reduces p62 accumulation, which exerts antifibrotic effects by inhibiting NF-κB-mediated profibrotic gene expression. Targeting the CD148 phosphatase with activating ligands such as SDC2-pep may represent a potential therapeutic strategy in IPF.
Keywords: CD148; fibroblast; idiopathic pulmonary fibrosis; nuclear factor-kappa-B; syndecan-2.
Figures






Comment in
-
How Do We Know What We Are Missing? Loss of Signaling through CD148 Drives Fibroblast Activation in Pulmonary Fibrosis.Am J Respir Crit Care Med. 2021 Aug 1;204(3):249-251. doi: 10.1164/rccm.202103-0737ED. Am J Respir Crit Care Med. 2021. PMID: 33891825 Free PMC article. No abstract available.
References
-
- Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. - PubMed
-
- Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. - PubMed
-
- Harrod TR, Justement LB. Evaluating function of transmembrane protein tyrosine phosphatase CD148 in lymphocyte biology. Immunol Res. 2002;26:153–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

